|
|
|
International Pharma and Medical Device Compliance Congress XII Features Annual CCO Roundtable; Early Bird Ends on 3/30
|
- Sponsored by ETHICS, the International Society of Healthcare Compliance Professionals
- Media Partner: Life Science Compliance Update
- May 14 - 16, 2018
- Onsite at the Hotel Savoyen Vienna, Austria
- Vienna, Austria
- www.InternationalPharmaCongress.com
PRESS RELEASE
Phone: 800-503-8171
Email: registration@hcconferences.com
Website: www.InternationalPharmaCongress.com |
EARLY BIRD REGISTRATION
- SAVE UP TO €400 -
|
Register by Friday, March 30, 2018 and save up to €400.
Click here to register.
|
|
|
FEATURING ANNUAL CHIEF COMPLIANCE OFFICERS ROUNDTABLE WITH
|

Julie Bonhomme
Legal Affairs and Compliance Deputy Director, European Federation of Pharmaceutical Industries and Associations (EFPIA), Brussels, Belgium |

Suzanne Durdevic, LLM
Senior Director, General Counsel Europe, Boston Scientific International; Chair, Legal Affairs Committee, MedTech Europe; Member, Strategic Committee, International Society of Healthcare, Ethics and Compliance Professionals (ETHICS), Paris, France |

Tomasz Kruk
Head of Compliance, Vifor Pharma; Former Director, International Compliance, Mallinckrodt Pharmaceuticals; Former Director, Global Ethics and Compliance, Actavis plc (now Allergan), Zurich, Switzerland
|

Evelyne Lemaire, MA
Head of Compliance, Europe and Canada, Takeda Pharmaceuticals International GmbH, Zurich, Switzerland |

Thomas J. Schumacher, JD
Vice President and Global Chief Ethics and Compliance Officer, Medtronic; Awardee, 2017 PwC MedTech Ethical Leadership Award, Fridley, MN, USA |

Mariusz Witalis
Partner, Fraud Investigation & Dispute Services, EY, Warsaw, Poland (Moderator)
|
|
|
|
SPECIAL RATES AVAILABLE FOR
|
 |
 |

|
 |
 |

|
|
VIENNA, AUSTRIA -- PHARMA UPDATE NEWS SERVICE™ -- MARCH 14, 2018: The Twelfth International Pharmaceutical and Medical Device Compliance Congress, www.InternationalPharmaCongress.com, will be held May 14 - 16, 2018 at the Hotel Savoyen, Vienna, Austria.
|
|
KEYNOTE SPEAKERS
|

Nicola Bedlington
Secretary General, European Patients Forum (EPF), Former Director, European Disability Forum, Brussels, Belgium |

Serge Bernasconi, MA, MIM
Chief Executive Officer, MedTech Europe, Former CEO, Eucomed, Former CEO, European Diagnostic Manufacturers Association, Brussels, Belgium |

Richard Bistrong, MA
Chief Executive Officer, Front-Line Anti-Bribery LLC, Contributing Editor, FCPA Blog, Former Confidential Human Source (CHS) and Cooperating Witness, FBI and US DOJ, Former Cooperating Witness, City of London Police, HMRC and CPS, UK, New York, NY, USA
|

Giovanni Buttarelli
European Data Protection Supervisor, Former Secretary General, Italian Data Protection Authority, Brussels, Belgium |

Charles Cain, JD
Chief, FCPA Unit, US Securities and Exchange Commission (SEC), Washington, DC, USA |

Paul Csiszár, JD
Director, DG Competition, European Commission, Brussels, Belgium
|

Tarek Helou, JD
Assistant Chief, FCPA Unit, Fraud Section, Criminal Division, US Department of Justice, Assistant US Attorney, Northern District of California, Washington, DC, USA |

Jan Oliver Huber, DR JUR
General Secretary, Association of the Austrian Pharmaceutical Industry (PHARMIG), Vienna, Austria |

José F. Zamarriego Izquierdo, MBA, PhD
Director, Code of Practice Surveillance Unit, Farmaindustria, Chair, EFPIA's Code Committee, Coordinator, EFPIA e4ethics Initiative, Chairman, IFPMA's Code Complaint Procedure Appeal Group, Madrid, Spain
|

George "Ren" McEachern, CFE, CAMS
Managing Director, Exiger, Former Supervisory Special Agent, International Corruption Squad, US Federal Bureau of Investigation, Washington, DC, USA |

Sofie Melis
Senior Manager, Ethics and Compliance, International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), Geneva, Switzerland |

Marie-Claire Pickaert
Deputy Director General, Chief Financial Officer and Ambassador to the Medical Communities, European Federation of Pharmaceutical Industries and Associations (EFPIA), Brussels, Belgium
|

Camilla de Silva
Joint Head of Bribery and Corruption, Serious Fraud Office, London, UK |

Heather Simmonds
Director, Prescription Medicines Code of Practice Authority, London, UK
|

Professor N. Craig Smith
Affiliate Professor of Ethics and Social Responsibility, and Chair, Ethics and Social Responsibility, INSEAD, Academic Director, CSR & Ethics Research Group, INSEAD Social Innovation Centre, Fontainebleau, France |

Tamara Tubin
International Compliance Director, Corporate Compliance, Wright Medical Group N.V., Vice Chair, Ethics & Compliance Committee, MedTech Europe, Member, Strategic Committee, International Society of Healthcare, Ethics and Compliance Professionals (ETHICS), Zürich, Switzerland
|
|
|
|
AGENDA-AT-A-GLANCE
|
|
Day I: Monday, May 14, 2018
|
- Morning Complimentary Precons
- Luncheon your Own
- Afternoon Opening Plenary Session
- Welcome PHARMIG and Co-Chairs
- C-suite Keynote
- OECD Update
- EU Commission Keynote
- US DOJ and SEC FCPA Update
- UK SFO Update
- Annual CCO Roundtable
- Evening Networking Reception
|
|
|
Day II: Tuesday, May 15, 2018
|
- Morning Plenary Session
- Global Compliance Codes Update
- Richard Bistrong Behind the Bribe
- Annual Anti-corruption Roundtable
- Networking Luncheon
- Afternoon Mini Summits: Round I
- Managing Third Party Relationships
- EU General Data Protection Privacy Update (GDPR)
- Future of Direct Sponsorships
- Patient Organizations (EFPIA White Paper)
- Annual MEA Compliance Update
|
|
|
Day II: continued
|
- Afternoon Mini Summits: Round II
- Outcomes-based Value-added Services
- Annual Medical Device Compliance Update
- Social Responsibility: EU Directive 2014/95/EU
- Evolution of Compliance Programs
- Annual CEE Compliance Update
- Afternoon Mini Summits: Round III
- Social Media Compliance
- Compliance Monitoring and Risk Assessments
- Relationships with HCPs, HCOs and Pt Organizations
- Behavioral Economics in Compliance Programs
- Compliance Issues in Market Access
|
|
Day III: Wednesday, May 16, 2018
|
- Morning Closing Plenary Session
- European Data Protection Supervisor Update
- IFPMA Update
- MedTech Europe Update
- Healthcare Compliance as a Profession
- Challenges and Opportunities of Pursuing the Healthcare Compliance Profession
- Embracing Change in the Digital Health Era
- The Ethics of Artificial Intelligence
- 12:30 pm Adjournment
|
|
|
FEATURED FACULTY
|

Ted Acosta, Esq.
Americas Vice Chair, Risk Management, EY; Former Senior Counsel, Office of Inspector General, United States Department of Health and Human Services, New York, NY, USA and Paris, France |

John Alexander
Head of European Compliance, Mundipharma International, Former EMEA Director of Compliance, Ortho Clinical Diagnostics, Former Healthcare Compliance Officer, Janssen-Cilag, Cambridge, UK |

David Andersen
Partner, Pharmaceuticals and Life Sciences, PwC, London, UK
|

Michael Bartke, PhD
Former Director Compliance Management, Daiichi Sankyo Europe GmbH, Strategic Committee, International Society of Healthcare, Ethics and Compliance Professionals (ETHICS), Munich, Germany |

Ann Beasley, JD
Director, Navigant Consulting, Former Senior Vice President, Chief Compliance Officer, Biogen, Former Co-chair, International Pharmaceutical \and Medical Device Compliance Congress, Boston, MA, USA |

Liesbeth Bergmans, LLM
Senior Legal Counsel, Teva Pharmaceuticals, Amsterdam, Netherlands
|

Lee Betteridge
Director, Pharmaceutical and Life Sciences, PwC, London, UK |

Julie Bonhomme
Legal Affairs & Compliance Deputy Director, European Federation of Pharmaceutical Industries and Associations (EFPIA), Brussels, Belgium |

Eduard Castells
Global Head Integrity and Compliance Policies, and Risk Assessment at Novartis, Novartis, Basel, Switzerland
|

Ceren Aral Desnos, LLM
Interim Director of Legal and Compliance (Consultant), MedTech Europe, Paris, France |

Holger Diener
Managing Director, Association of Voluntary Self-Regulation for the Pharmaceutical Industry ("FSA"), Member, Ethics and Compliance Committee and Vice Chair, Code Committee, EFPIA, Member, IFPMA Code Compliance Network, Berlin, Germany |

Peter Dieners, Esq.
Partner and Head, Global Healthcare and Life Sciences Group, Clifford Chance, Co-chair, Legal Affairs Focus Group (LA FG), EUCOMED, Co-chair, Compliance Network (CN), EUCOMED, Düsseldorf, Germany
|

Diva Duong
Global Compliance Solutions Partner, IQVIA, Paris, France |

Kip Ebel, MBA
Principal, Fraud Investigation & Dispute Services, EY, New York, NY, USA |

Sue Egan
Director and Principal Consultant, Sue Egan Associates, Former Vice President Compliance, AstraZeneca, Great Missenden, Buckinghamshire, UK
|

Olga Sanz Escudero
Compliance Advisor, Merck; Former Codes Compliance Officer, Farmaindustria, Madrid, Spain |

Karen Eryou
Head, Ethics & Compliance Programs, UCB Pharma, Former Co-chair, Asia Pacific Pharma Compliance Congress, Brussels, Belgium |

George Fife
Partner, Fraud Investigation & Dispute Services, EY, Former Executive Director, Compliance & Ethics, Bristol-Myers Squibb, Paris, France
|

Jacques Fontas
Executive Counsel, Compliance Leader Europe, GE Healthcare, Member, Strategic Committee, ETHICS, Paris, France |

Geert van Gansewinkel, MSc, MBA, IESE
Managing Partner, Polaris Europe and Asia-Pacific, Polaris BV, Amsterdam, Netherlands |

Gary F. Giampetruzzi, Esq.
Partner, Paul Hastings, Former Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc., New York, NY, USA
|

Cecile Gousset
Head of Compliance Risk Assessment, Policies & Education, Global Ethics & Business Integrity, Sanofi, Paris, France |

Ulf H. Grundmann
Partner, FDA and Life Sciences, King & Spalding LLP, Frankfurt am Main, Germany |

Joseph W. Henein, PharmD
President and Chief Executive Officer, NewBridge Pharmaceuticals FZ LLC, Dubai, UAE
|

Casey J. Horton, CFE
Director, Healthcare and Life Sciences Disputes, Regulatory, Compliance and Investigations, Navigant Consulting, Chicago, IL, USA |

Els Janssens, LLM
Senior Associate, Baker & McKenzie Habib Al Mulla, Former Legal Adviser, European Medicines Agency, Former Senior Legal Counsel, Johnson & Johnson, Abu Dhabi, UAE |

Carl Judge
Partner, Fraud Investigation & Dispute Services, EY, London, UK
|

Ilknur Kehlibar
Associate Director, Health Care Compliance, Turkey and Partner Markets, Celgene, Istanbul, Turkey |

Isaure Kergall-Gayet, DEA-Law, CAPA
Legal Director, France, Amgen, Former VP, Chief Ethics & Compliance Officer, Ipsen, Paris, France |

Maria Khoury
Compliance Officer Levant, Roche, Chair, Lebanese Group of International Pharmaceutical Companies, and Iraq LAWG(Local Area Working Group), Beirut, Lebanon
|

Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC, USA |

Tomasz Kruk
Head of Compliance, Vigor Pharma, Former Director International Compliance, Mallinckrodt Pharmaceuticals, Former Director Global Ethics & Compliance, Actavis plc (now Allergan), Zurich, Switzerland |

Anita Kyung-Hee Kim-Reinartz
Partner, Fraud Investigation & Dispute Services, EY, Düsseldorf, Germany
|

Laurence Labey, MD
Ethics and Business Integrity Business Partner for R&D and Chief Medical Office, Sanofi, Paris, France |

Evelyne Lemaire, MA
Head of Compliance, Europe & Canada, Takeda Pharmaceuticals International GmbH, Zurich, Switzerland |

Abdul Luheshi, MBA
Executive Director, Global Operations, Ethics & Compliance, Astellas Pharma EMEA, Former Vice President, Health Care Compliance, Johnson & Johnson, Chertsey, UK
|

John McLoughlin, JD
Counsel, Friedman & Wittenstein, Washington, DC |

Paul J. Melling, JD
Founding Partner, Baker & McKenzie – CIS, Limited, Moscow, Russia |

Laura Nassar
Head of Compliance Middle East, Roche Pharmaceuticals, Former Regional Pharma HCC Officer Emerging Markets, Johnson & Johnson, Beirut, Lebanon
|

Chrisoula Nikidis
Lead, Canada, Polaris; Former Executive Director, Ethics and Compliance, Innovative Medicines Canada; Former Industry Co-Chair Designate, APEC Biopharmaceutical Working Group on Ethics, Ottawa, Canada |

Nicola Orlandi
Head Data Privacy Pharma, Global Privacy Office, Novartis International AG, Former Head of Legal and Compliance, Sandoz S.p.A., Basel, Switzerland |

Giota Papamarkou
Global Ethics and Compliance Director, Ipsen, Former EMEA Compliance and Ethics Manager, Bristol-Myers Squibb, Paris, France
|

Oscar Perdomo
Manager, Pharmaceutical and Life Sciences, PwC, Basil, Switzerland |

Nathalie Pirlot
Third Party Compliance Management Lead, UCB, Brussels, Belgium |

Madina Plieva, PhD
Legal and Compliance Head, Russia, Bristol-Myers Squibb, Former Legal Director, Association of International Pharmaceutical Manufacturers (AIPM), Former Head of Legal Department, Kutafin Moscow State Law Academy, Moscow, Russia
|

Ethan M. Posner, JD
Partner, Covington, Former Deputy Associate Attorney General, US Department of Justice, Washington, DC, USA |

Uffe Kåre Rasmussen, MSc
Senior Director, Chief Compliance Officer, Corporate Compliance & Corporate Social Responsibility, H. Lundbeck A/S, Copenhagen, Denmark |

Ulrich Matthias Reese, Esq.
Partner and Co-Head Global Healthcare, Life Sciences and Chemicals Group, Clifford Chance; Chair of the Pharmaceutical Law Committee of the Association for Intellectual Property and Copyright Law (GRUR), Düsseldorf, Germany
|

Vivian Robinson, Esq.
Queen's Counsel, Partner, McGuire Woods, Former General Counsel of the UK Serious Fraud Office, Former Head, QEB Hollis Whiteman Chambers, Recorder of the Crown Court and Treasurer of Inner Temple, London, UK |

Fabien Roy
Counsel, Hogan & Lovells, Brussels, Belgium |

Christine M. Sainvil
Compliance Officer, EthicalMedtech, Manager, Conference Vetting System (CVS), European Medical Technology Industry Association (MedTech Europe), Brussels, Belgium
|

Thomas J. Schumacher, JD
Vice President and Global Chief, Ethics and Compliance Officer, Medtronic, Awardee, 2017 PwC MedTech Ethical Leadership Award, Fridley, MN, USA |

Brian Sharkey, JD
Vice President, Porzio Life Sciences, LLC, Principal, Porzio, Bromberg & Newman, Morristown, NJ, USA |

Hubertus Stockmann, MBA
Regional Compliance Officer EMEA, Getinge, Former Compliance Officer Performance Material & Merck Group Functions, Merck Group, Frankfurt Am Main, Germany
|

Michele Tagliaferri, JD
Partner and Lead, Investigations and Compliance Team, Sidley Austin LLP, Brussels, Belgium |

Yuet Ming Tham, JD
Partner, Sidley Austin LLP; Former Asia Pacific Regional Compliance Director, Pfizer; Former Deputy Public Prosecutor, Singapore, Hong Kong |

Evgeny Tilezhinsky, MBA, PhD
Law and Compliance Lead for the Central and Eastern Europe, Bristol-Myers Squibb, Uxbridge, Middlesex, UK
|

Dumitru Uta, MD, MBA, CCEP
Ethics and Compliance Director, South Eastern Europe, Eli Lilly and Company, Bucharest, Romania |

Pawel Wajszczyk, MBA
Director HCCO Eastern Europe, Russia, CIS MD, Johnson & Johnson, Warsaw, Poland
|

Mariusz Witalis
Partner, Fraud Investigation and Dispute Services, Ernst & Young LLP, Warsaw, Poland |

Christine Zettlemoyer, JD
Head, Americas Compliance and Global Support, CSL Behring, Former Executive/ Head, Global Compliance Oversight and Monitoring, Merck, King of Prussia, PA, USA
|
|
|
|
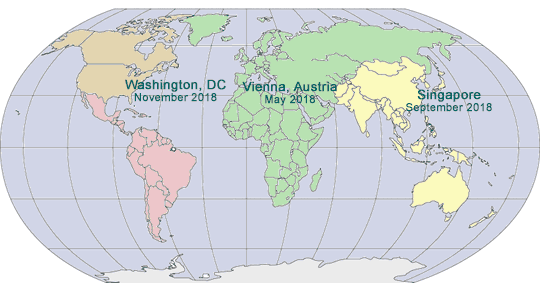
2018 GLOBAL PHARMA COMPLIANCE CONGRESSES
|
|
TWELFTH INTERNATIONAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
Sponsored by International Society of Healthcare Compliance Professionals (ETHICS)
Media Partners: Life Sciences Compliance Update
May 14 - 16, 2018
Hotel Savoyen
Vienna, Austria
www.International PharmaCongress.com
|
|
EIGHTH ASIA PACIFIC PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
Sponsored by Asia Pacific Healthcare Industry Compliance Team
Cosponsored by International Society of Healthcare Ethics and Compliance Professionals (ETHICS)
Media Partners: Life Sciences Compliance Update
September 10 - 12, 2018
Singapore
www.Asian PharmaCongress.com
|
|
NINETEENTH ANNUAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Pharmaceutical Compliance Forum (PCF)
November 7 - 9, 2018
Mandarin Oriental
Washington, DC
www.PharmaCongress.com
|
|
|
|
|
|
SPONSORED BY:
|

|
|
CO CHAIRS:
|

Dante Beccaria
Senior Vice President, Global Compliance Officer, Head of Ethics & Business Integrity Department, Sanofi, Paris, France

Stephen Nguyen Duc
Former Area Director, International Operations, Western Europe & Canada, Office of Ethics & Compliance, AbbVie Board Member, Strategic Committee International Society of Healthcare, Ethics, and Compliance Professionals (ETHICS), Rungis, France

Suzanne Durdevic, LLM
Senior Director, General Counsel Europe, Boston Scientific International, Chairs, Legal Affairs Committee, MedTech Europe, Member, Strategic Committee, International Society of Healthcare, Ethics and Compliance Professionals (ETHICS), Paris, France

Dominique Laymand, Esq.
Executive Vice President, Chief Ethics and Compliance Officer, Ipsen; Honorary President, International Society of Healthcare Ethics and Compliance Professionals (ETHICS), Paris, France

Roeland Van Aelst
Regional Vice President – HCCO MD&D EMEA & Canada, Office Health Care Compliance and Privacy, Johnson & Johnson, Chairman, International Society of Healthcare, Ethics and Compliance Professionals (ETHICS), Chairman, MedTech Compliance Network, Brussels, Belgium
|
|
GRANTORS:
|
|
DIAMOND
|

|
|
SILVER
|


|
|
BRONZE
|





|
|
MEDIA PARTNER:
|

|
|
REGISTRATION DISCOUNTS AVAILABLE TO
|






|
|
CONTINUING EDUCATION CREDITS:
|
Accounting Professionals: Approved for up to 19.0 NASBA CPE credits.
Attorneys: Pending approval for Pennsylvania CLE credits.
Compliance Professionals: Pending Compliance Certification Board (CCB) approval of CEUs.
Click here for more information.
|
|
FOLLOW INTERNATIONAL PHARMA CONGRESS ON
|


Tweet using #PharmaCongress
|
|
INTERNATIONAL PHARMA CONGRESS IS
|

|
|
OTHER INTERNATIONAL PHARMA CONGRESSES
|

2007 - Brussels

2008 - Paris

2009 - Rome

2010 - Berlin

2007 - Brussels

2008 - Paris

2011 - Singapore

2012 - Budapest

2012 - Shanghai

2012 - São Paulo

2013 - Madrid

2013 - Kuala Lumpur

2014 - Dubai

2014 - Mexico City

2014 - Shanghai

2015 - Manila

2016 - Warsaw

2017 - Lisbon
|
|
|
|
|