|
|
|
Press Release: Tenth International Pharma Compliance Congress Agenda and Brochure Now Available
|
- A Hybrid Conference and Internet Event
- Sponsored by ETHICS, the International Society of Healthcare Compliance Professionals
- Co-sponsored by the Pharmaceutical Compliance Forum
- May 10 - 12, 2016
- Onsite at the Hilton Warsaw Hotel and Convention Centre in Warsaw, Poland
- In Your Own Office or Home live via the Internet with 24/7 Access for Six Months
- www.InternationalPharmaCongress.com
PRESS RELEASE
Phone: 800-503-8171
Email: registration@hcconferences.com
Website: www.InternationalPharmaCongress.com |
EARLY BIRD REGISTRATION
- SAVE UP TO €400 -
|
Register by Friday, March 18, 2016 for Early Bird Discount and save up to €400.
Click here to register.
|
|
|
WARSAW, POLAND -- PHARMA UPDATE NEWS SERVICE™ -- FEBRUARY 23, 2016: The Tenth International Pharmaceutical Compliance Congress, www.InternationalPharmaCongress.com, will be held on May 10 - 12, 2016 at the Hilton Warsaw Hotel and Convention Centre in Warsaw, Poland.
|

|
KEYNOTE SPEAKERS
|

Sadek Beloucif, MD
Professor, Department of Anesthesiology and Critical Care Medicine, Avicenne University Hospital, Chairman, Ethics Committee of the Biomedicine Agency, Paris, France |

Hui Chen, JD
Compliance Counsel Expert, US Department of Justice, Former Global Head, Anti-Bribery and Corruption, Standard Chartered Bank, Former Assistant General Counsel, Pfizer, Washington, DC, USA |

Peter Dieners, Esq.
Partner and Head, Global Healthcare and Life Sciences Group, Clifford Chance, Co-chair, Legal Affairs Focus Group (LA FG), EUCOMED, Co-chair, Compliance Network (CN), EUCOMED, Düsseldorf, Germany
|

Gejaa Gobena, JD
Chief, Health Care Fraud Unit, US Department of Justice, Washington, DC, USA |

Ewa Grenda
Chief Executive Officer, Poland Roche, President, INFARMA, Warsaw, Poland |

Jose F. Zamarriego Izquierdo
Director Unidad de Supervision Deontologica, FARMAINDUSTRIA, Madrid, Spain
|

Dr. Krzysztof Landa
Vice Minister of Health in Charge of Drug Policy, Polish Ministry of Health; Founder, Watch Health Care Foundation, Warsaw, Poland |

Sophie Peresson
Director, Pharmaceuticals & Healthcare Programme, Transparency International UK, London, UK |

Marie-Claire Pickaert
Deputy Director General and Chief Financial Officer, European Federation of Pharmaceutical Industries and Associations (EFPIA), Brussels, Belgium
|

Heather Simmonds
Director, Prescription Medicines Code of Practice Authority, London, UK |

Pawel Sztwiertnia
Chief Executive Officer, Employers' Union of Innovative Pharmaceutical Companies (INFARMA), Former Undersecretary of State, Polish Ministry of Health, Warsaw, Poland
|
|
|
|
EFPIA CODES TRAINING WORKSHOP
|
 The European Federation of Pharmaceutical Industries and Associations (EFPIA) is offering an independent EFPIA CODES TRAINING WORKSHOP on Tuesday, May 10, 2016.
The European Federation of Pharmaceutical Industries and Associations (EFPIA) is offering an independent EFPIA CODES TRAINING WORKSHOP on Tuesday, May 10, 2016.
- What can we learn from each other?
- Come and find out practical cases to help improve your understanding of the Codes, their implementation by national associations and global requirements.
- Each section will include a presentation of a relevant case from national associations and a description of the relevant requirements from the IFPMA and/or EFPIA Codes, as well as the national codes.
Click here for more information.
|
|
|
CHIEF COMPLIANCE OFFICERS
|

Timothy Ayers, JD, MPH
Vice President, Chief Compliance Officer, Horizon Pharma plc, Former VP, Chief Compliance Officer, Dendreon, Former Associate General Counsel and Executive Director of Compliance, Seattle Genetics, Chicago, IL, USA |

Ann Beasley
Chief Compliance Officer, Biogen, Former Global Compliance Officer, Novartis Pharma AG, Board Member and Co-chair, Strategic Committee International Society of Healthcare, Ethics, and Compliance Professionals (ETHICS), Cambridge, MA, USA |

Dante Beccaria
Global Compliance Officer and Vice President, Sanofi, Former Vice President of Internal Audit, Sanofi, Paris, France
|

Eric Cornut, LLM
Chief Ethics, Compliance & Policy Officer, Novartis, Former Chief Commercial Officer, Novartis, Basel, Switzerland |

Martin Fürle, MBA (Berlin)
General Counsel and Chief Compliance Officer, Vice President Risk & Compliance, Daiichi Sankyo Europe GmbH, Munich, Germany |

Marc-Olivier Lamaro
Chief Compliance Officer, Sanofi Pasteur MSD, Former Director, Compliance Officer, Johnson & Johnson, Lyon, France
|

Dominique Laymand, Esq.
Senior Vice President, Chief Ethics and Compliance Officer, Ipsen; President, International Society of Healthcare Ethics and Compliance Professionals (ETHICS), Paris, France |

Uffe Kåre Rasmussen, M.Sc.
Senior Director and Chief Compliance Officer, H. Lundbeck A/S, Former Principal Scientist, Novo Nordisk A/S, Copenhagen, Denmark
|

Ashley Watson, Esq.
Senior Vice President, Chief Ethics & Compliance Officer, Merck Sharp Dohme (MSD), Former Counsel, Chief Ethics and Compliance Officer, Hewlett-Packard, Philadelphia, PA, USA |

Ilya Yuffa, MBA
Vice President, Global Ethics and Compliance Officer, Eli Lilly and Company, Indianapolis, IN, USA
|
|
|
|
GLOBAL ANTICORRUPTION UPDATE
|

Ted Acosta, Esq.
Americas Vice Chair, Risk Management and Global Leader, Life Sciences, Fraud Investigation and Dispute Services, EY, Former Senior Counsel, Office of Inspector General, US Department of Health and Human Services, New York, NY, USA and Paris, France |

Cristian Ducu, PhD
Founder and General Manager, Centre for Advanced Research in Management and Applied Ethics, Member, National Bioethics Commission of the National Commission of Romania for UNESCO, Founding Member, Research Centre in Applied Ethics, Faculty of Philosophy, University of Bucharest, Bucharest, Romania |

Gary F. Giampetruzzi, Esq.
Partner, Paul Hastings, Former Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc., New York, NY, USA
|

Michael K. Loucks, Esq.
Partner, Skadden Arps LLP, Former Acting United States Attorney, District of Massachusetts, United States Department of Justice, Washington, DC, USA |

Paul J. Melling, Esq.
Founding Partner, Baker & McKenzie - CIS, Limited, Moscow, Russia |

Vivian Robinson, Esq.
Queen's Counsel, Partner, McGuire Woods, Former General Counsel of the UK Serious Fraud Office, Former Head, QEB Hollis Whiteman Chambers, Recorder of the Crown Court and Treasurer of Inner Temple, London, UK
|
|
|
|
THE NEW PHARMA MARKETPLACE
|

Lee Betteridge
Director, Pharmaceuticals and Life Sciences, PwC, London, UK |

Patrick Delavault, MD
Senior Vice President, Chief Medical Officer, Global Medical and Regulatory Sciences, Ipsen, Paris, France |

Maarten Meulenbelt, Esq.
Partner, Food, Drug, and Medical Device Regulatory Group, Sidley Austin LLP, Brussels, Belgium
|

Frank Wartenberg, PhD
President, Central Europe, IMS Health, Frankfurt/Main, Germany |

Andrew White
Head of Medicine Management - NHS, Greater Manchester Commissioning Support Unit, Manchester, UK |

Olivier Wong
Consultant, Early Engagement, HTA Technology Transfer, MediQualité Omega, Former Voting Member, French Transparency Committee, Former French Guidelines Committee Member (HAS, ANSM), Paris, France
|
|
|
|
PERSPECTIVES ON TRANSPARENCY FROM POLAND
|

Professor Jacek Jassem, MD, PhD
Head, Department of Oncology and Radiotherapy, Medical University of Gdansk; Former President, Polish Society of Oncology, Gdansk, Poland |

Dr. Krzysztof Landa
Vice Minister of Health in Charge of Drug Policy, Polish Ministry of Health; Founder, Watch Health Care Foundation, Warsaw, Poland
|

Dr. Grzegorz Makowski
Director of the Public Integrity Program, Stefan Batory Foundation, the Local Partner with Transparency International in Poland, Warsaw, Poland |

Pawel Sztwertnia
Director General, INFARMA; Former Undersecretary of State, Polish Ministry of Health, Warsaw, Poland
|
|
|
|
PLENARY SESSIONS
|
|
|
- Overview of the EU, CEE and MEA Anti-Corruption Landscape
- The National Anti-Corruption Strategy of Romania
- US Department of Justice Anti-Corruption Update
- Anti-Corruption Roundtable: Strategies to Foster Compliance
- Challenges in the New Marketplace 2.0 Ethics and the Current Industry-Physician Relationship Models
- EFPIA Keynote: Reflections on the Eve of Transparency
- Perspectives on Transparency from Poland
- Transparency International Keynote
- Chief Compliance Officer Roundtable
- Report from Compliance Survey Commissioned by Ethics
- Updates on Global Codes
|
INTERNATIONAL PHARMA
CONGRESS IS
|

|
|
|
|
MINI SUMMITS
|
- Examining an Investigation from All Sides
- Annual CEE Compliance Update
- New Strategies for Integrated Assessment and Management of Risks
- Patient Programs - Managing Risks of Getting Closer to the Patient
- Annual MEA Compliance Update
- Contracting with Third Parties and Optimizing the Relationship
- Introduction of a New Product into the New Marketplace
- Data Privacy Case Study: Transferring Data Post the EJC Safe Harbour Ruling
- Cultural Implications of Compliance Programs across Different Countries
- Using Predictive Analytics to Enhance Risk Management
- Third Party Relationships: Cross-Border Engagements, FMV, Monitoring and Auditing
- New Ethics-Based Approaches to Policies and Training
- Medical Devices: The Changing Enforcement Landscape
- R&D Compliance: Case Study on Clinical Trial Data Disclosures
- Using the Data to Provide Value and Partner with the Business
|
|
|
|
FEATURED FACULTY
|

Anthony Alvizu, CPA, EnCE, CFE
Senior Director, Global Risk & Investigations Practice, FTI Consulting, Chicago, IL, USA |

David Andersen
Partner, Pharmaceuticals and Life Sciences, PwC, London, UK |

Yogesh Bahl, CPA, MBA
Managing Director, Alix Partners, New York, NY
|

Marc Bartel, LLM, MBA
Managing Partner France, Partner, Legal and Board Practices, Heidrick & Struggles, Paris, France |

Andy Bender, MS, MBA
President and Founder, Polaris, New York, NY |

Ela Bochenek, Esq.
Vice President, Global Compliance, Insmed Incorporated, Bridgewater, NJ, USA
|

Julie Bonhomme, Esq.
Legal Affairs and Compliance Deputy Director, European Federation of Pharmaceutical Industries and Associations (EFPIA), Brussels, Belgium |

Olivier Chaduteau
Managing Partner, Day One, Mentor for Entrepreneurs, IME (French Chamber of Commerce), Lecturer, Sciences Po Law School, Paris, France |

Tanya Daniels, Esq.
Senior Legal Director, EU Head of Data Protection, Deputy Global Chief Privacy Officer, Quintiles, Reading, UK
|

Zeynep Didem Degirmencioglu, LLM
Regional Head of Group Compliance, Intercontinental Region, Merck Serono, Istanbul, Turkey |

Pierre E. Dupourque, JD
Assistant General Counsel, Regional Compliance Lead EMEA, Canada, Pakistan, India, Pfizer, Mannheim, Germany |

Kip Ebel, MBA
Principal, Fraud Investigation & Dispute Services, EY, New York, NY, USA
|

Sue Egan
Director and Principal Consultant, Sue Egan Associates, Former Vice President Compliance, AstraZeneca, Great Missenden, Buckinghamshire, UK |

George Fife
Executive Director, Fraud Investigation & Dispute Services, EY, Former Executive Director, Compliance & Ethics, Bristol-Myers Squibb, Paris, France |

Elvan Sevi Firat, LLM, PhD
Founding Partner, Firat - Izgi Attorney Partnership, Istanbul, Turkey
|

Cécile Gousset
Associate Vice President, Compliance Risk Assessment, Education and Monitoring, Global Ethics & Business Integrity, Sanofi, Paris, France |

Ulf H. Grundmann
Partner, Life Science Practice Group, King & Spalding LLP, Frankfurt am Main, Germany |

Saul Helman, MD, MBA
Managing Director and Life Sciences Practice Leader, Navigant Consulting, Inc., Indianapolis, IN, USA
|

Joe Henein
President and Chief Executive Officer, NewBridge Pharmaceuticals, Former Regional Managing Director, Middle East, and North Africa, Wyeth Pharmaceuticals, Dubai, UAE |

Carl Judge
Director, Fraud Investigation & Dispute Services, EY, London, UK |

Suzana Junaci, MD
Ethics and Compliance Officer, Eli Lilly and Company, Zagreb, Croatia
|

Anita K. Kim-Reinartz
Partner, Fraud Investigation & Dispute Services, EY, Düsseldorf, Germany |

Isaure Kergall-Gayet
Compliance and Business Integrity Director France, Sanofi, Members, Strategic Committee, ETHICS, Former Vice President, Chief Ethics and Compliance Officer, Ipsen, Paris, France |

Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC
|

Tomasz Kruk, MBA, LLM
Head of Compliance, Vifor Pharma, Former Director International Compliance, Mallinckrodt Pharmaceuticals, Former Director Global Ethics & Compliance, Actavis plc (now Allergan plc), Zürich, Switzerland |

Laurence Labey
Research and Development Compliance, Sanofi, Paris, France |

Edoardo Lazzarini, PhD
Compliance Initiative Director EUCAN, Takeda Pharmaceuticals International, Former Compliance Officer EMEA (Europe, Middle East & Africa), Biomet, Milano, Italy
|

Evelyne Lemaire, MA
Head of Compliance, Europe & Canada, Takeda Pharmaceuticals International GmbH, Zurich, Switzerland |

Christine Longawa, CPA, CFE
Associate Director, Healthcare & Life Sciences Disputes, Regulatory, Compliance and Investigations, Navigant Consulting, Inc.. Chicago, IL, USA |

Maurits J.F. Lugard, MA, JD, LLM
Partner and Lead, EU Life Sciences Regulatory Team, Sidley Austin LLP, Former Member of the European Commission's Legal Service, Brussels, Belgium
|

Abdul Luheshi, MBA
Director, Life-Sciences, Regulatory Compliance, KPMG UK LLP, Former Vice President, Health Care Compliance, Johnson & Johnson, Former Co-chair, Asia Pacific Pharma Compliance Congress, London, UK |

Daniela Fabian Masoch, Esq.
Founder and Managing Director, Fabian Privacy Legal GmbH, Member and Chapter Co-Chair, International Privacy Professionals Association (IAPP), Former Global Head Data Privacy, Novartis International AG, Basel, Switzerland |

Maureen McGirr, Esq.
Vice President Office of Ethics, Global Compliance Organization, Merck Sharp Dohme (MSD), Philadelphia, PA, USA
|

Laura Nassar
Head of Compliance, Middle East, Hoffmann-La Roche Ltd., Dubai, UAE |

George K. Ng, Esq.
Executive Vice President and Chief Legal Officer, Sorrento Therapeutics, Inc., San Diego, CA, USA |

John Patrick Oroho, Esq.
Executive Vice President and Chief Strategy Officer, Porzio Life Sciences, LLC, Principal, Porzio, Bromberg & Newman PC, Morristown, NJ, USA
|

David O'Shaughnessy
Vice President, Compliance, Emerging Markets, Quintiles, Board Member, ETHICS, Former Vice President Compliance, International Pharmaceuticals, GSK, Former Vice President, Global Compliance Strategy, AstraZeneca, Reading, UK |

Giota Papamarkou
Global Ethics & Compliance Director, Ipsen, Former EMEA Compliance and Ethics Manager, Bristol-Myers Squibb, Paris, France |

Piergiorgio Pepe, LLM
EEMEA Compliance Area Director, Office of Ethics and Compliance, AbbVie, Former Director, Compliance and Ethics, EMEA, Bristol-Myers Squibb, Paris, France
|

Oscar Perdomo
Director, Pharmaceuticals and Life Sciences, PwC, Switzerland |

Maria Teresa Rico Perez, MD
Europe and Canada Regional Compliance Officer, Biogen, Zug, Switzerland |

Katalin Pungor, MD, PhD, MBA
Director, Healthcare Compliance, Emerging Markets and CEE, Johnson & Johnson, Budapest, Hungary
|

Ariadna Quesada, MPP
Compliance Manager Europe, Middle-East, and Africa, MicroPort Orthopedics Inc., Amsterdam, Netherlands |

Nathalie Raynaud
Corporate Compliance Officer, Ethics & Business Integrity Department, Sanofi, Paris, France |

Abdallah El Saadi, CCP
Regional Compliance Officer - Middle East, Turkey and Africa, Boehringer Ingelheim, Dubai, UAE
|

Daniel Schafaghi
Global Compliance Operating Officer, Head of Compliance Emerging Markets, Boehringer Ingelheim GmbH, Ingelheim, Germany |

Ben Shoshan
Director/Owner, Consultant and Executive Coach, Open Water, London, UK |

Hubertus Stockmann, MBA
Divisional Compliance Officer, Merck KgaA, Frankfurt, Germany
|

Agata Szeliga, LLM
Partner, Soltysinski, Kawecki and Szelzak, Warsaw, Poland |

Michele Tagliaferri, Esq.
Partner, Sidley Austin LLP, Brussels, Belgium |

Melda Tanyeri, MS, MBA
Director, Fraud Investigation & Dispute Services, EY, London, UK
|

Dr. Mirjam Weisse, JD
Senior Director, Head of Compliance EMEA, Merz Pharma GmbH & Co, KGaA, Frankfurt, Germany |

Mariusz Witalis
Partner, Fraud Investigation and Dispute Services, Ernst & Young LLP, Warsaw, Poland |

Elisabethann Wright, Esq.
Partner, Hogan Lovells, Former Senior Legal Officer and Hearing Officer, EFTA Surveillance Authority, Brussels, Belgium
|

Jolanta Wyszynska
Vice President Compliance Europe, AstraZeneca, Warsaw, Poland |

Milena Yakimova, LLM
Director Ethics & Compliance, Central and South East Europe, Allergan, Sofia, Bulgaria
|
|
|
|
|
|
SPONSORED BY:
|

|
|
CO-SPONSORS:
|


|
|
CO CHAIRS
|

Dr. Michael Bartke
Director Compliance Management, DAIICHI SANKYO EUROPE GmbH, Munich, Germany
|

Ann Beasley
Chief Compliance Officer, Biogen, Former Global Compliance Officer, Novartis Pharma AG, Board Member and Co-chair, Strategic Committee International Society of Healthcare, Ethics, and Compliance Professionals (ETHICS), Cambridge, MA, USA
|

Stephen Nguyen Duc
Office of Ethics & Compliance Area Director, International Operations, Western Europe & Canada, AbbVie, Board Member, Strategic Committee International Society of Healthcare, Ethics, and Compliance Professionals (ETHICS), Rungis, France
|

Dominique Laymand, Esq.
Senior Vice President, Chief Ethics and Compliance Officer, Ipsen; President, International Society of Healthcare Ethics and Compliance Professionals (ETHICS), Paris, France
|

Roeland Van Aelst
Regional Vice President - HCCO MD&D EMEA & Canada, Office Health Care Compliance and Privacy, Johnson & Johnson, Co-chair, Strategic Committee, International Society of Healthcare, Ethics and Compliance Professionals (ETHICS), Chairman, MedTech Compliance Network, Brussels, Belgium
|
|
GRANTORS:
|
|
DIAMOND
|

|
|
SILVER
|

|
|
BRONZE
|









|
|
CONTINUING EDUCATION CREDITS
|
Accounting Professionals: Approved for up to 19 NASBE CPE credits.
Compliance Professionals: The Congress is currently pending approval to offer Compliance Certification Board CCB Credits.
Attorneys: The Congress is currently pending approval to offer California and Pennsylvania MCLE Credit.
Click here for more information.
|
|
FOLLOW INTERNATIONAL PHARMA CONGRESS ON
|


Tweet using #PharmaCongress
|
|
OTHER INTERNATIONAL PHARMA CONGRESSES
|

2007 - Brussels

2008 - Paris

2009 - Rome

2010 - Berlin

2011 - Istanbul

2011 - Singapore

2012 - Budapest

2012 - Shanghai

2012 - São Paulo

2013 - Madrid, Spain

2013 - Kuala Lumpur

2014 - Dubai

2014 - Mexico City

2014 - Shanghai

2015 - Manila
|
|
PARTICIPATION OPTIONS
|
|
|
|
TRADITIONAL ONSITE ATTENDANCE
|
Simply register, travel to the conference city and attend in person.
Pros: subject matter immersion; professional networking opportunities; faculty interaction

|
|
LIVE AND ARCHIVED WEBCAST PARTICIPATION
|
Watch the conference in live streaming video over the Internet and at your convenience at any time 24/7 for the six months following the event.
The archived conference includes speaker videos and coordinated PowerPoint presentations.
Pros: Live digital feed and 24/7 Internet access for next six months; Accessible in office, at home or anywhere worldwide with Internet access; Avoid travel expense and hassle; No time away from the office


|
WEBCAST INTERFACE SAMPLE
|
Click here for a sample stream
|
THE 2015 NINTH INTERNATIONAL PHARMA CONGRESS CONTENT IS NOW AVAILABLE IN VARIOUS POST CONFERENCE FORMATS
|
|
The 2015 Ninth International Pharma Congress conference content is now available in a variety of formats.
You may purchase the Congress streaming content in the following formats: Flash Drive or online archive (6 months). You may also purchase individual presentations in an online archive (6 months) format.
YOU CAN PURCHASE JUST THE INTERNATIONAL PHARMA CONGRESS CONTENT AS FOLLOWS:

Online Archive of 2015 Ninth International Pharma Congress Presentations today!
Complete conference: $595
Order Now
|

Flash Drive of 2015 Ninth International Pharma Congress Presentations today!
$595
Order Now
|
FINALLY YOU MAY PURCHASE INTERNATIONAL PHARMA CONGRESS INDIVIDUAL PRESENTATIONS:
|
Click here to purchase individual presentations for $59.95 in online archive format (6 months of access - 24/7).
|
|
|
|
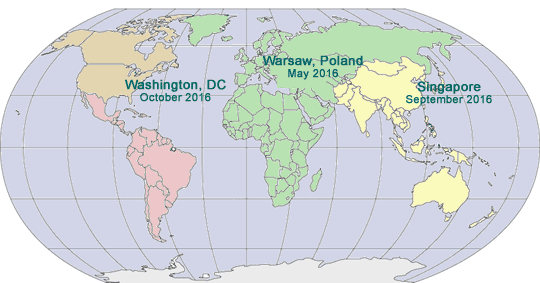
2016 GLOBAL PHARMA COMPLIANCE CONGRESSES
|
|
SEVENTEENTH PHARMACEUTICAL REGULATORY AND COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Transformational Learning - Effective Knowledge Exchange
Sponsored by Pharmaceutical Compliance Forum
Media Partners: Harvard Health Policy Review, Health Affairs and Life Sciences Compliance Update
October 19 - 21, 2016
Mandarin Oriental
Washington, DC
www.PharmaCongress.com
|
|
SIXTH ASIA PACIFIC
PHARMACEUTICAL
COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Asia Pacific Healthcare Industry Compliance Team
Cosponsored by International Society of Healthcare Ethics and Compliance Professionals (ETHICS) and Pharmaceutical Compliance Forum (PCF)
Media Partners: Life Sciences Compliance Update
September 21 - 23, 2016
Shangri-La Hotel
Singapore
www.AsianPharmaCongress.com
|
|
PHARMACEUTICAL SUMMIT ON BUSINESS AND COMPLIANCE ISSUES IN MANAGED MARKETS
|
The Leading Forum on Pharmaceutical Market Access, Reimbursement, Pricing, and Contracting with Commercial and Government Payers
Co-located with Eighth National Medical Home, Sixth Bundled Payment Summit and Seventh National ACO Summit
Media Partners: Harvard Health Policy Review, Health Affairs and Life Sciences Compliance Update
June 8 - 9, 2016
Grand Hyatt
Washington, DC
www.PharmaManagedMarketsSummit.com
|
|
|
|
|
|
|