PRESS RELEASE: New Hungarian Pharmaceutical Code of Ethics to be Announced at the Sixth International Pharmaceutical Compliance Congress

- A Hybrid Conference and Internet Event
- Sponsored by the Pharmaceutical Compliance Forum
- May 14 - 16, 2012
- Onsite at the Hilton Budapest, Budapest, Hungary
- Online In Your Own Office or Home live via the Internet with 24/7 Access for Six Months
- www.InternationalPharmaCongress.com
PRESS RELEASE
Phone: 800-503-8171
Email: registration@hcconferences.com
Website: www.InternationalPharmaCongress.com
WASHINGTON DC USA -- PHARMA UPDATE NEWS SERVICE™ -- APRIL 18, 2012: The International Pharmaceutical Compliance Congress, www.InternationalPharmaCongress.com, will take place on May 14 - 16, 2012 at the Hilton Budapest in Budapest, Hungary. The Congress will be offered both onsite and live and archived for 6 months over the Internet.

|
KEYNOTE SPEAKER
|

Christopher A. Viehbacher
Chief Executive Officer, Sanofi, Paris, France
|
|
|
|
|
|
FEATURED SESSIONS
|
|
International Pharma Congress Vision and Overview
The Role of Global Compliance in the Pharmaceutical Enterprise
EU Annual Compliance and EFPIA Compliance Initiatives Update
CEE Annual Compliance Update
MEA Annual Compliance Update
Regional Anti-Bribery Developments Roundtable: US FCPA, UK Bribery Act, Russian Anti-Bribery Act, OECD Anti-Bribery Convention
E4ethics Congresses European Assessment Platform
Regional Transparency and Disclosure Initiatives: UK, US, Netherlands, France, Slovak Republic
Global Transparency Management: Industry Survey Key Findings
Regional Compliance Code Update Roundtable: IFPMA, EFPIA, EUCOMED, MEA, Russia, Turkey, PhRMA
Third-party Intermediaries: Due Diligence and Monitoring Considerations
Business Ethics and Reputation in the Pharmaceutical and Medical Device Enterprises
|
|
|
PHARMA COMPLIANCE TRACKS
|
|
Central and Eastern Europe Compliance Update
Middle East and Africa Compliance Update
Israel Compliance Update
Global Anti-Bribery Update
Global Compliance Audits and Monitoring
Global Transparency, Discourse and Aggregate Spend
New EU Provisions Governing Compliance with Pharmaceutical Regulatory Laws
The Italian Regulatory and Compliance Scene in Depth: A Case Study
Asia Pacific Regulatory and Compliance Scene in Depth: A Case Study
Lessons with Global Compliance Issues and Initiatives in the Medial Device Sector
|
|
|
OTHER KEYNOTE FACULTY
|

Bulent Becan
Managing Partner, Atuva Management Consultancy and Trade Ltd.; Consultant in Ethics Issues, Turkish Research Based Pharmaceutical Companies Association (AIFD), Istanbul, Turkey |

Richard Bergström
Director General, European Federation of the Pharmaceutical Industries and Associations (EFPIA), Former Director General, LIF Sweden, Brussels, Belgium |

Joe Henein
President and Chief Executive Officer, NewBridge Pharmaceuticals; Former Regional Managing Director, Middle East and North Africa, Wyeth Pharmaceuticals, Dubai, United Arab Emirates
|

Jose F. Zamarriego Izquierdo
Director Unidad de Supervision Deontologica, FARMAINDUSTRIA, Madrid, Spain |

Andrew Jack
Pharmaceuticals Correspondent, Financial Times, London, UK |

Aline Lautenberg
Senior Legal Counsel, Eucomed, Brussels, Belgium
|

Bernard Maillet, MD
Member, Flemish Chamber of the Board for Specialists for Pathology, Treasurer, VBS-GBS, Member, EUCOMED's Compliance Panel, Former Secretary General, UEMS and EACCME, Antwerp, Belgium |

Tamara Music
Manager, Influenza Vaccines & Code Compliance, IFPMA, Geneva, Switzerland |

Marie-Claire Pickaert
Deputy Director General, European Federation of Pharmaceutical Industries and Associations (EFPIA), Brussels, Belgium
|

Andy Powrie-Smith
Director for Trust and Reputation, Association of the British Pharmaceutical Industry (ABPI), London, UK |

Heather Simmonds
Director, Prescription Medicines Code of Practice Authority, London, UK |

Sona Strachotova, MBA
Executive Director, Slovak Association of Research-based Pharmaceutical Companies (SAF), Bratislava, Slovak Republic
|

Madina Torchinova, Esq.
Regional Compliance Officer CEE, Sandoz; Former Director Legal and Regulatory Affairs, Association of International Pharmaceutical Manufacturers (AIPM), Russia, Munich, Germany |

Matthijs M. van Blokland, Esq.
General Counsel, Prosensa, General Counsel and Senior Policy Advisor Legal Affairs, Association Innovative Medicines Nefarma, Board Member, Stichting CGRz, Amsterdam, The Netherlands
|
|
|
|
ANTICORRUPTION UPDATE: FCPA UK BRIBERY ACT, RUSSIAN ANTI-BRIBERY,
OECD ANTI-BRIBERY CONVENTION
|

Ted Acosta, Esq.
Principal, Ernst & Young LLP; Former Senior Counsel, Office of Inspector General, US Department of Health and Human Services, New York, NY, USA and Paris, France |

John T. Bentivoglio, Esq.
Partner, Skadden Arps LLP; Former Special Counsel for Healthcare Fraud and Chief Privacy Officer, US Department of Justice, Washington, DC |

Michael J.J. Brueck, LLM, Ph.D. (Dr. iur.)
Counsel, Sidley Austin LLP, Professor of Law, German Graduate School of Management and Law, Heilbronn, Germany, Frankfurt, Germany
|

Raymond Emson, Esq.
Policy Lawyer, Serious Fraud Office, London, UK |

Gary F. Giampetruzzi, Esq.
Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc., New York, NY, USA |

Edward F. Hanover, III
General Counsel, Novo Nordisk Region International Operations A/S, Zurich, Switzerland
|

Rosanne M. Kay, Esq.
Partner, Reed Smith, London, UK |

Paul J. Melling, Esq.
Founding Partner, Baker & McKenzie - CIS, Limited, Moscow, Russia
|

Vivian Robinson, Esq.
Partner, McGuireWoods; Former General Counsel of the UK Serious Fraud Office; Former Head, QEB Hollis Whiteman Chambers; Recorder of the Crown Court and Treasurer of Inner Temple, London, UK |

Joseph B. Tompkins, Jr.
Partner, Sidley Austin LLP; Former Deputy Chief of the Fraud Section, Criminal Division, United States Department of Justice, Washington, DC, USA
|
|
|
|
GLOBAL TRANSPARENCY, DISCLOSURE AND AGGREGATE SPEND
|

Ronny Arijs
Vice President Global Sustainability and Chief Compliance Officer, Grunenthal; Member, EFPIA Compliance Workgroup; Former Senior Compliance Manager, GE Healthcare, Brussels, Belgium |

Yogesh Bahl, MBA
Partner, National Practice Leader - Life Sciences, Deloitte Financial Advisory Services LLP, New York, NY, USA |

Michael Bartke, PhD
Director, Compliance Management, Internal Audit and Corporate Compliance, Daiichi Sanky Europe GmbH, Vice Chair, EFPIA Compliance Committee, Munich, Germany
|

Julie Bonhomme, Esq.
Legal Manager, LEEM, Paris, France |

William E. Buzzeo, MS
Vice President and General Manager, Global Compliance Solutions, Cegedim Relationship Management, Richmond, VA, USA |

Fred Eaton
Partner, Polaris Management Partners, New York, NY, USA
|

Kelly B. Freeman, PhD
Ethics and Compliance Officer, Eli Lilly and Company, Indianapolis, IN, USA |

Jonathon Kellerman
Principal, Pharmaceutical and Life Sciences Advisory Services, PricewaterhouseCoopers LLP, Florham Park, NJ, USA |

Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC, USA
|

John Patrick Oroho, Esq.
Executive Vice President, Porzio Pharmaceutical Services; Principal, Porzio Bromberg & Newman PC, Morristown, NJ, USA |

David Wysocky
Director, Pharmaceutical and Life Sciences Advisory Services, PricewaterhouseCoopers LLP, Boston, MA, USA
|
|
REGIONAL COMPLIANCE CODE UPDATE: IFPMA, EFPIA, EUCOMED,
MEA, RUSSIA, TURKEY
|

Peter Dieners, Esq.
Partner, Clifford Chance, Düsseldorf, Germany |

Michael K. Loucks, Esq.
Partner, Skadden Arps LLP; Former Acting United States Attorney, US Attorney's Office for the District of Massachusetts, Boston, MA, USA
|
|
|
BUSINESS ETHICS AND REPUTATION
|

Andy Powrie-Smith
Director for Trust and Reputation, Association of the British Pharmaceutical Industry (ABPI), London, UK |

Paul B. Woods, BPharm, MA, MRPharmS
Independent Consultant; Former Global Compliance Policy Director, AstraZeneca, Macclesfield, Cheshire, UK
|
|
|
CENTRAL AND EASTERN EUROPEAN COMPLIANCE UPDATE
|

Rozalia Gyulai
Regulatory Manager, Pfizer, Budapest, Hungary |

Tomasz Kruk, LL M, MBA
Director Global Ethics and Compliance, Actavis, Zug, Switzerland
|

Paul J. Melling, Esq.
Founding Partner, Baker & McKenzie - CIS, Limited, Moscow, Russia |

Mariusz Witalis
Partner, Fraud Investigation and Dispute Services, Ernst & Young LLP, Warsaw, Poland
|
|
|
ISRAEL COMPLIANCE UPDATE
|

Daniel Kessler
Principal, ITR Group, Former Chief Counsel, Int'l Trade Controls, Pfizer, Former Chief Counsel, Int'l Trade Controls, Wyeth, Tel Aviv, Israel |

Guy Schmidt, MBA
Compliance Director, AstraZeneca (Israel) Ltd., Tel Aviv, Israel
|
|
|
MIDDLE EAST/AFRICA COMPLIANCE UPDATE
|

Hulya Baran
Director, Ethics & Compliance TMEA - CIS, Eli Lilly and Company, Istanbul, Turkey |

Sameh Farag
Associate Vice President Global Compliance, Intercontinental Zone Global Compliance, Sanofi, Paris, France |

Meltem Ozker Gunduz
Regional Legal Counsel and Compliance Officer, Business Area Near East, Novo Nordisk, Istanbul, Turkey
|

Liz MacGillivray
Compliance Director, Corporate Integrity and Compliance, Novartis International AG, Basel, Switzerland |

Laura Nassar
Regional Pharma HCC Officer Emerging Markets, Johnson & Johnson, Beirut, Lebanon
|
|
|
GLOBAL COMPLIANCE AUDITING AND MONITORING BEST PRACTICES
|

Antoinette Gutierrez-Crespin
Partner, Ernst & Young, Paris France |

Stefan Heissner
Managing Partner, Fraud Investigation and Dispute Services, Ernst & Young GmbH, Düsseldorf, Germany |

Luca M. Liberatore
Director Large European Affiliates and CEE, Amgen International HealthCare Compliance, Milan, Italy
|

Michael J. Morgan
Ethics and Compliance Officer, Australia, Canada and Europe, Eli Lilly and Company, Windlesham, Surrey, United Kingdom |

Dave O'Shaughnessy
Vice President, Global Compliance, Astra-Zeneca; Former Vice President and Compliance Officer, Emerging Markets and Asia Pacific, GlaxoSmithKline, London, UK |

L. Stephan Vincze, JD, LL M, MBA
National Managing Director, Life Sciences/Forensic and Dispute Services, Deloitte Financial Advisory Services, LLP, Boston, MA, USA
|
|
|
DUE DILIGENCE AND MONITORING THIRD PARTY INTERMEDIARIES
|

John Alexander
Head of European Compliance, Mundipharma International, Former EMEA Director of Compliance, Ortho Clinical Diagnostics, Ormer Healthcare Compliance Officer, Janssen-Cilag, Cambridge, United Kingdom |

Karl Boonen, Esq.
Senior Director Business Practices and Compliance EMEA and Canada, Johnson & Johnson, Antwerp, Belgium
|

Nieves Liste, MBA
Global Business Compliance Director, Covidien, Barcelona, Spain |

Marc L. Miller
Partner, KPMG LLP, New York, NY, USA
|
|
NEW EU PROVISIONS GOVERNING COMPLIANCE WITH
PHARMACEUTICAL REGULATORY LAWS
|

Sue Egan
Director and Principal Consultant, Sue Egan Associates; Editor, Life Science Compliance; Former Vice President Compliance, AstraZeneca, Great Missenden, Buckinghamshire, UK |

Evelyn Lemaire
Director, PricewaterhouseCoopers LLP, Zurich, Switzerland |

Maurits J.F. Lugard, MA, JD, LL M
Partner, Sidley Austin LLP; Former Member of the European Commission's Legal Service, Brussels, Belgium
|

Paul B. Woods, BPharm, MA, MRPharmS
Independent Consultant; Former Global Compliance Policy Director, AstraZeneca, Macclesfield, Cheshire, UK |

Elisabethann Wright, Esq.
Partner, Hogan Lovells; Former Senior Legal Officer and Hearing Officer, EFTA Surveillance Authority, Brussels, Belgium
|
|
|
THE ITALIAN REGULATORY AND COMPLIANCE SCENE IN DEPTH
|

Maurizio Arena, Esq.,
Criminal Lawyer, President and Founding Member, Osservatorio 231 Farmaceutiche, Rome, Italy |

Maurizio Bortoletti
Colonel, Carabinieri Corps, Extraordinary Commissioner, Public Healthcare Company, Salerno, Italy |

Maria Teresa Brassiolo
President and Founding Member, Transparency International Italia, Milan, Italy
|

Antonio Cavallaro
Compliance & Internal Auditing Senior Manager, Takeda Italia Farmaceutici, Rome, Italy |

Edoardo Lazzarini, PhD
European Compliance Officer, Biomet Italia, Milan, Italy |

Giuseppe Palmieri
Chief Compliance Officer, Head of Risk & Compliance Management and Internal Audit, Boehringer Ingelheim Italia, Milan, Italy
|
|
|
THE CHINESE REGULATORY AND COMPLIANCE SCENE IN DEPTH
|

Erinn Hutchinson
Director, PricewaterhouseCoopers LLP, Philadelphia, PA, USA |

Yuet-Ming Tham, Esq.
Partner, Sidley Austin LLP, Former Regional Compliance Director, Legal Division, Corporate Compliance, Pfizer Inc., Hong Kong
|

George Ng, Esq.
Partner, Snell & Wilmer; Former Head of Legal, Chief Compliance Officer, Chief IP Counsel, Spectrum Pharmaceuticals, Inc.; Former Manager, Intellectual Property, Alpharma Inc., Costa Mesa, CA |

John Auerbach, MA
Partner, Fraud Investigation and Dispute Services, Ernst & Young China, Shanghai, China
|
|
|
GLOBAL MEDICAL DEVICE SECTOR COMPLIANCE ISSUES
|

Brian Riewerts
Partner, Global Pharmaceuticals and Life Sciences, PricewaterhouseCoopers LLP, Baltimore, MD, USA |

Sujata T. Dayal, Esq.
Corporate Vice President and Chief Compliance Officer, Biomet; Former Counsel, Domestic Legal Operations, Abbott Laboratories; Former Member, PCF Executive Committee, Warsaw, IN, USA
|

Peter V. Rother, Esq.
Associate General Counsel and Senior Compliance Director, Cardiovascular Division, St. Jude Medical, Plymouth, MN, USA |

Tamara Tubin
Director Ethics and Compliance International, CareFusion International, Former Associate Director Compliance EMEA, ZIMMER GmbH, Zürich, Switzerland
|
|
|
|
|
|
|
SPONSORED BY:
|
|

The Pharmaceutical Compliance Forum (PCF) is a coalition of senior compliance professionals and legal counsel from more than 50 of the largest research-based pharmaceutical manufacturers. The PCF was founded in early-1999 by compliance professionals from the pharmaceutical industry to promote effective corporate compliance programs. The members meet twice a year, for two days, focusing on open and informal sharing of compliance information, best practices, and current developments in the field, and sponsors a two-day international compliance congress in the Spring and a three-day US compliance congress each Fall.
|
|
|
THE INTERNATIONAL PHARMA CONGRESS IS DEDICATED TO THE MEMORY OF
|

Gabor Danielfy
Vice-President & Global Compliance Officer, Sanofi
Former Senior Director Healthcare Compliance Europe, Middle East & Africa, Johnson & Johnson
Former Senior Director Global Compliance & Business Practices EMEA, Schering Plough Corp
Former General Manager Andean Region, Bayer Pharmaceuticals
Founder, ETHICS (EMEA Think-tank on Healthcare Integrity and Compliance Strategies)
Member, EFPIA Code Steering Group
Member, IFPMA Code Network
Co-Chair, International Pharma Compliance Congress
Paris, France
|
|
CLICK HERE TO VIEW GABOR'S COMMENTS ON THE ROLE OF COMPLIANCE IN THE GLOBAL PHARMACEUTICAL ENTERPRISE
|
|
|
|
GABOR DANIELFY MEMORIAL CONCERT
|

The family of Gabor Danielfy invite you to a memorial concert to be on Wednesday, May 16, 2012 at 2 pm at the Institute for Musicology of the Hungarian Academy of Sciences, the Bartok room, only 50 meters from the Hilton Budapest. The concert will feature a performance of the piano music of Liszt.
|
|
CONGRESS CO CHAIRS
|

Ann Beasley Bacon
Global Compliance Officer, Novartis Pharma AG, Basel, Switzerland
|

Kelly B. Freeman, PhD
Ethics and Compliance Officer, Eli Lilly and Company, Executive Committee Member, Pharmaceutical Compliance Forum, Indianapolis, IN, USA
|

Dominique Laymand, Esq.
Vice President Compliance & Ethics EMEA (Europe, Middle-East, Africa, Russia and Turkey), Bristol-Myers Squibb, Paris, France
|

Roeland Van Aelst
Vice President EMEA & Canada, J&J Office Health Care Compliance & Privacy, Johnson&Johnson, Brussels, Belgium
|
|
|
MEDIA PARTNERS
|


|
|
GRANTORS:
|
|
DIAMOND
|

|
|
PLATINUM
|

|
|
GOLD
|

|
|
SILVER
|


|
|
BRONZE
|







|
|
ONLINE ATTENDEE LOG IN
|
Attendees registered for the International Pharma Congress Online Video/Audio Archive - click here to log in.
|
|
|
|
FEATURING VIDEO PRESENTATIONS BY GLOBAL PHARMA CEOs:
|
|
2011 Fifth International Pharma Congress Keynote Address by
|

David Brennan
Chief Executive Officer, AstraZeneca, President, IFPMA, Past Chairman, PhRMA, London, UK
|
|
2012 Twelfth Pharmaceutical Regulatory Compliance Congress and Best Practices Forum by
|
John C. Lechleiter, PhD
Chairman, President and Chief Executive Officer, Eli Lilly and Company, Chairman-elect, PhRMA, Indianapolis, IN
|
|
INTERNATIONAL PHARMA CONGRESS PLANNING COMMITTEE
|
Ann Beasley Bacon
Global Compliance Officer, Novartis Pharma AG, Basel, Switzerland (Co chair)
Kelly B. Freeman, PhD
Ethics and Compliance Officer, Eli Lilly and Company, Indianapolis, IN, USA (Co chair)
Dominique Laymand, Esq.
Vice President Compliance & Ethics EMEA (Europe, Middle-East, Africa, Russia and Turkey), Bristol-Myers Squibb, Paris, France (Co chair)
Roeland Van Aelst
Vice President EMEA & Canada, J&J Office Health Care Compliance & Privacy, Johnson&Johnson, Brussels, Belgium (Co chair)
Ted Acosta, Esq.
Principal, Ernst & Young LLP, Former Senior Counsel, Office of Inspector General, US Department of Health and Human Services, New York, NY, USA and Paris, France
Ronny Arijs
Vice President Global Sustainability & Chief Compliance Officer, Grunenthal, Former Senior Compliance Manager GE Healthcare, Brussels, Belgium
Yogesh Bahl, MBA
Partner, National Practice Leader - Life Sciences, Deloitte Financial Advisory Services LLP, New York, NY, USA
Wayne Baker
Senior Vice President & Chief Sales Officer, Advanced Health Media LLC, New Providence, NJ, USA
Bulent Becan
Managing Partner, Atuva Management Consultancy and Trade Ltd., Consultant in Ethics Issues, Turkish Research Based Pharmaceutical Companies Association (AIFD), Istanbul, Turkey
John T. Bentivoglio, Esq.
Partner, Skadden Arps LLP, Former Special Counsel for Healthcare Fraud, and Chief Privacy Officer, US Department of Justice, Washington, DC, USA
Hugh C. Bigwood
Ethics and Compliance Officer, International Operations, Abbott Laboratories, Horsham, West Sussex, United Kingdom
Antonio Cavallaro
Compliance & Internal Auditing Senior Manager, Takeda Italia Farmaceutici, Rome, Italy
Peter Dieners, Esq.
Partner, Clifford Chance, Düsseldorf, Germany
Pierre E. Dupourque
Regional Compliance Director, Corporate Compliance, International Investigations and Programs, Pfizer Inc, Mannheim, Germany
Sue Egan
Director and Principal Consultant, Sue Egan Associates, Editor, Life Science Compliance, Former Vice President Compliance, AstraZeneca, Great Missenden, Buckinghamshire, UK
Annette Schutt Fiig
Director, Risk Office, Novo Nordisk, Copenhagen, Denmark
Gerard Geneen
Vice President, Compliance Officer, Pharma Europe, GlaxoSmithKline, Brentford, Middlesex, United Kingdom
Sameh Farag
Associate Vice President Compliance, Intercontinental Zone Corporate Compliance, Sanofi, Paris, France
Gary F. Giampetruzzi, Esq.
Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc, New York, NY, USA
Jonathon Kellerman
Principal, Pharmaceutical and Life Sciences Advisory Services, PricewaterhouseCoopers LLP, Florham Park, NJ, USA
Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC, USA
Marc L. Miller
Partner, KPMG LLP, New York, NY, USA
Maxine Nogard
Senior Director, Global Corporate Compliance, Biogen Idec Inc., Weston, MA, USA
Dave O'Shaughnessy
Vice President, Global Compliance, AstraZeneca, Former Vice President and Compliance Officer, Emerging Markets & Asia Pacific, GlaxoSmithKline, London, United Kingdom
Guillaume Roussel
Vice President, Compliance Solutions EMEA, Cegedim, Paris, France
Joseph B. Tompkins, Jr.
Partner, Sidley Austin LLP, Former Deputy Chief of the Fraud Section, Criminal Division of the United States Department of Justice, Washington, DC, USA
Paul B. Woods, BPharm, MA, MRPharmS
Independent Consultant, Former Global Compliance Policy Director, AstraZeneca, Macclesfield, Cheshire, UK
Elisabethann Wright, Esq.
Partner, Hogan Lovells, Former, Senior Legal Officer and Hearing Officer, EFTA Surveillance Authority, Brussels, Belgium
|
|
|
PAST INTERNATIONAL PHARMA CONGRESSES
|

2007 - Brussels, Belgium

2008 - Paris, France

2009 - Rome, Italy

2010 - Berlin, Germany

2011 - Istanbul, Turkey

2011 - Singapore
|
|
|
FOLLOW INTERNATIONAL PHARMA CONGRESS ON
|
|
|
INTERNATIONAL PHARMA CONGRESS IS
|

|
|

|
|
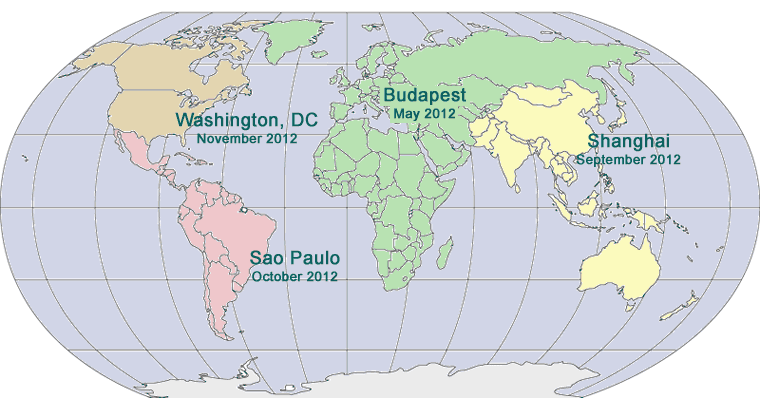
SAVE THE DATE
|
|
FOURTH ANNUAL SUMMIT ON DISCLOSURE, TRANSPARENCY AND AGGREGATE SPEND FOR DRUG, DEVICE AND BIOTECH COMPANIES
|
A Hybrid Conference and Internet Event
March 26 - 28, 2012
Renaissance Hotel
Washington, DC
www.DisclosureSummit.com
|
|
SECOND ASIA PACIFIC
PHARMACEUTICAL COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Pharmaceutical Compliance Forum and Asia Pacific Healthcare Industry Compliance Team
September 11 - 13, 2012
Intercontinental Shanghai Pudong Hotel
Shanghai, China
www.AsianPharmaCongress.com
|
|
FIRST LATIN AMERICAN PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Pharmaceutical Compliance Forum and Latin American Ethics and Compliance Network
October 2 - 4, 2012
Hilton Sao Paolo Morumba
Sao Paulo, Brazil
www.LatinAmericanPharmaCongress.com
|
|
THIRTEENTH PHARMACEUTICAL REGULATORY AND COMPLIANCE CONGRESS
|
Sponsored by Pharmaceutical Compliance Forum
November 5 - 7, 2012
Grand Hyatt
Washington, DC
www.PharmaCongress.com
|
|
|
NEW PARTICIPATION OPTION: LIVE AND ARCHIVED INTERNET ATTENDANCE
|
|
Announcing a new way to participate in the International Pharma Congress: You can now register to watch the Congress in live streaming video over the Internet and at your convenience at any time 24/7 for the six months following the event.
|
|
|
TRADITIONAL ONSITE ATTENDANCE
|
|
Simply register, travel to the conference city and attend in person.
Pros: subject matter immersion; professional networking opportunities; faculty interaction
|
|
|
|
LIVE AND ARCHIVED INTERNET ATTENDANCE
|
|
Watch the conference in live streaming video over the Internet and at your convenience
at any time 24/7 for the six months following the event.
The archived conference includes speaker videos and coordinated PowerPoint presentations.
Pros: Live digital feed and 24/7 Internet access for next six months; Accessible in office, at home or anywhere worldwide with Internet access; Avoid travel expense and hassle; No time away from the office
|
|
|
INTERNET INTERFACE SAMPLE
|
Click here for a sample stream
|
|
|
|



