|
|
|
FCPA Update by US DOJ & SEC Featured at the Virtual 15th Int'l Pharma and Medical Device Ethics & Compliance Congress
|
- Virtual Online Video Event Live and Archived
- Sponsored by International Society of Healthcare Compliance Professionals (ETHICS)
- Media Partner: Policy & Medicine Compliance Update
- May 17-20, 2022
- www.InternationalPharmaCongress.com
PRESS RELEASE
Phone: 800-503-8171
Email: registration@hcconferences.com
Website:
www.InternationalPharmaCongress.com
|
|
EARLY BIRD RATE
|
|
Click here to register by March 25 for early bird discount.
|
|
NEW REGISTRATION RATES FOR A VIRTUAL AGE
|
Registration discounts now available with special ETHICS/EFPIA/FSA/IFPMA/MedTech Europe/PCF rates. Rates are up to 1,000 euros less than major competing events.
- Click Here -
|
|
|
FEATURING THE ANNUAL FCPA ENFORCEMENT UPDATE BY
|

Robert I. Dodge, JD
Assistant Director, FCPA Unit, US Securities & Exchange Commission, Former Assistant Section, Environmental Defense Section, US Department of Justice, Washington, DC |

Derek J. Ettinger, JD, PhD
Assistant Chief, FCPA Unit, Fraud Section, Criminal Division, US Department of Justice, Washington, DC, USA |

Gary F. Giampetruzzi, JD
Partner, Paul Hastings; Former VP and Assistant General Counsel, Head of Investigations, Pfizer Inc. New York, NY, USA (Moderator)
|
|
PARIS, FRANCE -- PHARMA UPDATE NEWS SERVICE™ -- MARCH 16, 2022: The Virtual Fifteenth International Pharmaceutical and Medical Device Ethics and Compliance Congress, www.InternationalPharmaCongress.com, May 17 - 20, 2022, will take place in the form of online video broadcast which will be offered live and archived for 6 months following the live broadcast.
|
|
KEYNOTE SPEAKERS
|

Pablo Rojas Abad, LLM
Senior Legal Counsel, MedTech Europe, Etterbeek, Belgium |

Julie Bonhomme, Esq.
Legal & Compliance Director, European Federation of Pharmaceutical Industries and Associations (EFPIA), Brussels, Belgium |

Robert I. Dodge, JD
Assistant Director, FCPA Unit, US Securities & Exchange Commission, Former Assistant Section, Environmental Defense Section, US Department of Justice, Washington, DC, USA
|

Derek J. Ettinger, JD, PhD
Assistant Chief, FCPA Unit, Fraud Section, Criminal Division, US Department of Justice, Washington, DC, USA |

Antoinette Gawin, MSC
President & Chief Executive Officer, Terumo BCT; Chair, AdvaMed Board Ethics & Healthcare Compliance Committee, Denver, CO, USA |

Anna-Elisabeth Krause-Ablass, JD
European Delegated Prosecutor, European Public Prosecutor's Office; Former Specialized Public Prosecutor for White Collar Crime Frankfurt, Frankfurt, Germany
|

Dominique Laymand, Esq.
Of Counsel, Clifford Chance; Former Executive Vice President, Ethics & Social Responsibility Officer, Ipsen; Honorary President, ETHICS, Paris, France |

Oliver Medill
Managing Director, All About Impact;
Author, The Impact Formula: Powerful Solutions for Turbo-charging your Influence, London, UK |

Sofie Melis, MA
Director HR and Ethics & Compliance, International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), Geneva, Switzerland
|

Piergiorgio Pepe, MA. EU Law
President, Quantum Ethics, Ethics and Compliance Lecturer, SciencesPo; Board Member ETHICS, Paris, France |

David Shore
Faculty, Harvard University; Faculty, The Governance Institute; Advisory Board, McKinsey & Company; Professor of Innovation and Change, Tianjin University (China) & University of Monterrey, Business School (Mexico), Cambridge, MA, USA |

Julie Ritchie Wagner, JD
Senior Assistant General Counsel, PhRMA; Former Senior Counsel, Office of Counsel to the Inspector General, US Department of Health and Human Services, Washington, DC, USA
|
|
|
|
FEATURED FACULTY
|

Imelda Alvarez, LLB, MBA
Chief Executive Officer, Comply Latam, SC; Former Regional Integrity & Compliance Head, Latin America and Canada, Novartis, Mexico City, Mexico
|

Enno Behrendt, JD
Associate Director, Governance, Risk Management & Compliance, Guidehouse; Former Head of Compliance Diagnostic, Imaging and Services, Siemens Health, Nuremberg, Germany
|

Eric Bolesh
Chief Operating Officer, Cutting Edge Information, Raleigh-Durham, NC, USA
|

Kathleen M. Boozang, JD, LLM
Dean and Professor of Law, Seton Hall University School of Law, Fellow, The Hastings Center, Newark, NJ, USA
|

Kirsten Bröckers, MBLT
Head Legal & Compliance EMEA, Novartis Gene Therapies; Former Head Legal & Compliance EMEA, AveXis; Former Head of Legal and Compliance, Vifor Fresenius Medical Care Renal Pharma, Basel, Switzerland
|

Maija Burtmanis, LLB/BSc LLM
General Counsel & Chief Compliance Officer, Zuellig Pharma; Regional Compliance Director, Japan/Asia Pacific, Shire, Singapore
|

Antonio Cavallaro
Compliance Officer & Supervisory Body, Merck Serono Italy, Rome, Italy
|

Stéphanie Chabin, Esq.
General Counsel, Global R&D, Ferring; Former VP Legal R&D, Ipsen, Copenhagen, Denmark
|

Ana Christian, JD
Assistant General Counsel, Global Functional Practice Group, Genentech, Los Angeles, CA, USA
|

Campbell Clark, LLB, MJ
Vice President, Legal & Compliance, APAC, Medtronic, Covidien Private Limited; Chair, Compliance Committee, APACMed, Singapore
|

Zachary N. Coseglia, JD
Managing Principal, Head of Innovation and Co-Lead. R&G Insights LAB, Ropes & Gray LLP; Former Assistant General Counsel, Pfizer, Boston, MA
|

Audrey DeGuarde, MSJ
Vice President Global Transparency, Porzio Life Sciences, New York, NY
|

Ario Dehghani, JD
Of Counsel and Head, Compliance and Investigation Practice Group, Baker & McKenzie, CIS, Limited, Kyiv, Ukraine
|

Peter Dieners, Esq.
Regional Managing Partner (Germany) and Head, Global Healthcare and Life Sciences Group, Clifford Chance, Dusseldorf, Germany
|

Gildas Durand
Partner/Principal, Ernst & Young LLP, Miami, FL, US
|

Sue Egan, MBA
Director, Sue Egan Associates; Board and Co-chair, Strategic Committee, ETHICS, Great Missenden, UK
|

Jacob Elberg, JD
Associate Professor, Seton Hall University School of Law; Former Assistant US Attorney, US Attorney's Office, District of New Jersey, Newark, NJ, USA
|

Steffen Esche
Director, Consulting, Risk & Regulatory, PwC, Frankfurt, Germany
|

George Fife
Partner, Forensic & Integrity Services, EY; Former Executive Director, Compliance & Ethics, Bristol-Myers Squibb, Paris, France
|

Christopher J. Foreman, Esq.
Deputy Chief Privacy Officer, Merck Sharp & Dohme (Europe) Inc., Brussels, Belgium
|

Juan Luis Fuentes, Esq.
Ethics, Risk & Compliance, Head Latin America & Canada, Oncology, Novartis; Former Head of Legal and Compliance, Sandoz, East Hanover, NJ, USA
|

Elvira Valverde Garcia, LLM
Global Privacy Counsel, F.Hoffmann-La Roche Ltd; Former Corporate Counsel, Baxter International Inc., Zurich, Switzerland
|

Gary F. Giampetruzzi, JD
Partner, Paul Hastings; Former VP and Assistant General Counsel, Head of Investigations, Pfizer Inc. New York, NY, USA
|

Joe Henein
President and Chief Executive Officer, NewBridge Pharma; Former Regional Director, MEA, Wyeth, Dubai, UAE
|

Michael "Bret" Hood, MBA
CEO, 21st Century Learning & Consulting LLC; Former FBI Special Agent; Former Leadership Instructor, FBI's National Academy, Hillsborough, NC, USA
|

Bella Hovhannisyan, Esq.
Chief Ethics & Compliance Officer, CSL Behring AG; Member, ETHICS, Berne, Switzerland
|

Albert Hurtado
Global Customer Capabilities Manager, Amgen, Zurich, Switzerland
|

Els Janssens, LLM
Counsel, Baker & McKenzie, Brussels, Belgium
|

Vasiliki Kalaitzidis, JD
Vice President, Deputy General Counsel, Karyopharm Therapeutics Inc., Newton, MA, USA
|

Elisabeth Kohoutek, JD
Counsel, FDA and Life Sciences, King & Spalding, Frankfurt, Germany
|

Aline Lautenberg, Esq.
General Counsel and Director, Legal & Compliance, MedTech Europe, Brussels, Belgium
|

Cheryl Lee, MBA
Vice President, Global Market Compliance and Compliance Committees, Bristol-Myers Squibb; Former Vice President, Worldwide Markets, Healthcare Compliance, Celgene, Summit, NJ, USA
|

Gareth Lee, JD
Chief Compliance Officer & Asia Pacific General Counsel, Cordis; Former VP, Legal & Compliance, Asia Pacific, Cardinal Health, Singapore
|

Lei Li, LLM
Managing Partner, Beijing and Shanghai Offices, Sidley Austin; Former Third Secretary, Ministry of Commerce, People's Republic of China, Beijing, China
|

Michael K. Loucks, JD
Partner, Skadden Arps LLP; Former Acting US Attorney, District of Massachusetts, Washington, DC, USA
|

Desiree Maier, JD
Partner, Compliance and Investigations, Hogan Lovells; Lecturer, University of Augsburg, Munich, Germany
|

Inigo de la Maza, MSc, MBA
Data Scientist Solution Architect, F.Hoffmann-La Roche Ltd, Basel, Switzerland
|

Paul J. Melling, JD
Founding Partner, Baker & McKenzie, CIS, Limited, Moscow, Russia
|

Antje Meyer, LLM
Senior Manager, Forensic & Integrity Services, EY, Dusseldorf, Germany
|

Genevieve Michaux, LLM
Partner, FDA and Life Sciences, King & Spalding, Brussels, Belgium and Paris, France
|

Philippa Montgomerie, LLB
Vice President, Legal & Compliance, Medtronic, Edinburgh, Scotland, UK
|

Lena Moran-Adams, Esq.
Group Deputy General Counsel, Telix Pharma; Former Head of Legal and Business Conduct, Intercontinental Region, Gilead; Former Global Head of Legal, PLS, Novartis, Melbourne, Australia
|

Arthur Muratyan, Esq.
Secretary General, ETHICS; Former Chair, MedTech Compliance Panel, Paris, France
|

Jean-Claude Najar, JD, LLM
Compliance & ADR, Jean-Claude Najar Consultancy; Chair, MedTech Europe Compliance Panel, Paris, France
|

John Patrick Oroho, JD
Executive VP & Chief Strategy Officer, Porzio Life Sciences, LLC; Principal, Porzio Bromberg & Newman, PC, Morristown, NJ, USA
|

Rita Pan, MPH
Strategy Insights and Planning Associate Consultant, ZS, New York, NY, USA
|

Giota Papamarkou
Vice-President, Business Ethics North America & Global Monitoring, Ipsen; Former EMEA Compliance and Ethics Manager, BMS, Paris, France
|

Minal Patel, MS
Health Care Compliance Officer, Pharma and Consumer, Johnson & Johnson; Representative, Code Technical Advisory Committee, Johannesburg, South Africa
|

Oscar Perdomo
Director, Pharmaceutical & Life Sciences, Advisory Services, PwC Switzerland, Zurich, Switzerland
|

Nicole Peter
Global Compliance Leader, F.Hoffmann-La Roche Ltd, Zurich, Switzerland
|

Sergio Pinto, MBA
Sr. Director Compliance, Third Party Ethics & Compliance, Americas, Johnson & Johnson; Committee Member, Compliance Council Brasil, São Paulo, Brazil
|

Jenny Pu, CPA, CFE
Associate Director, Guidehouse Europe; Member, ETHICS, London, UK
|

Maria Eugenia (Maru) Quindimil, MBA
CEO and Founder, Socrates Compliance Consulting; Former Executive Director JAPAC, Amgen, Waianae, HI, USA
|

Maria Teresa Cantu Reus
Legal Advisor and Compliance Officer, FIFARMA; External Compliance Officer, AMIIF, Mexico City, Mexico
|

Michael L. Shaw, JD
Principal, Global Head of Risk & Compliance, ZS; Former VP & Compliance Officer, GSK, Philadelphia, PA, USA
|

Robert Stephenson
Director, PwC UK, London, UK
|

Geeta Thakerar, JD
Founder, Geeta Thakerar Consultancy Services; Former Chief Legal Counsel, Asia Pacific, Kimberly Clark, Singapore
|

Tamara Tubin, lic.iur.
Director, Ethics & Healthcare Compliance, Corporate, Johnson & Johnson; Board and Strategic Committee, ETHICS, Zurich, Switzerland
|

Elisabethann Wright, LLB
Partner, Cooley LLP, Brussels, Belgium
|

Mariusz Witalis
Partner, Forensic & Integrity Services, EY, Warsaw, Poland
|

Hady Zohdy
Ethics & Business Integrity Head for Egypt and Sudan, Sanofi; Chair, Egyptian Society For Pharmaceutical Researches (ESPR), Compliance Committee, Cairo, Egypt
|
|
|
|
|
|
SPONSORED BY:
|

|
|
CO CHAIRS
|

Anne-Sophie Bricca
Deputy General Counsel & Senior Director Legal Affairs & Compliance, Terumo BCT; Chair, Ethics and Compliance Group and seats at the Code Committee, MedTech Europe; Brussels, Belgium
|

Stephen Nguyen Duc
Global Head of Ethics & Compliance and Executive Counsel, GE Healthcare Pharmaceutical Diagnostics; Board Member, Strategic Committee International Society of Healthcare Ethics and Compliance Professionals (ETHICS); Paris, France
|

Laura Nassar, PharmD
Vice President, Head of Ethics & Business Integrity, AEME Region, Sanofi; Former Head of Compliance Middle East, Roche Pharmaceuticals; Former Regional Pharma HCC Officer Emerging Markets, Johnson & Johnson; Beirut, Lebanon
|

Roeland Van Aelst
EMEA Lead, Third Party Intermediary, Ethics & Compliance, Johnson & Johnson; President, International Society of Healthcare, Ethics and Compliance Professionals (ETHICS); Chairman, MedTech Europe Code Committee; Brussels, Belgium
|
|
GRANTORS
|
|
GOLD
|

|
|
SILVER
|



|
|
BRONZE
|












|
|
EXHIBITOR
|


|
|
CONTINUING EDUCATION
|
Compliance Certification Board (CCB)®.
The International Congress is pending approval from the Compliance Certification Board (CCB)(r) to offer preapproved Live and Non-Live CCB CEUs.

Continuing Legal Education
The International Congress is pending approval to offer Pennsylvania CLE Credits.

Certificates of Attendance
Attendees may download and self-complete a certificate of attendance which will be posted on the Congress virtual video broadcast platform to file with appropriate certifying bodies which accept such.
|
|
TUITION SCHOLARSHIPS
|
The International Congress is now offering a limited number of partial and full Tuition Scholarships to qualifying government representatives, consumer advocate organizations, academics, students, representatives of health services research organizations, and those who have lost their jobs as a result of the COVID-19 pandemic.
Click here for more information.
|


|
|
|
|
|
CONGRESS HIGHLIGHTS
|
- Live and curated sessions streamed directly to you through our easy to navigate advanced virtual broadcast platform
- New content, optimized for online learning, focused on new regulatory developments and managing ethics and compliance programs during the COVID 19 pandemic
- Engage with faculty through polling, Q & A sessions, chat and direct video messaging
- Network in real time with other attendees through direct attendee to attendee and group video messaging
- Access to an extensive video archive of past Congress presentations providing a context for the evolution of the compliance professions and the legal and regulatory setting
- Virtual exhibit hall featuring innovative solutions, products and services with opportunity to directly contact exhibitors for further information
|
|
|
|
REGISTRATION DISCOUNTS AVAILABLE FOR MEMBERS OF
|
 |
 |

|
 |
 |

|
|
|
FEATURING THE INTERNATIONAL CONGRESS ADVANCED VIRTUAL STREAMING PLATFORM
|

|
|
|
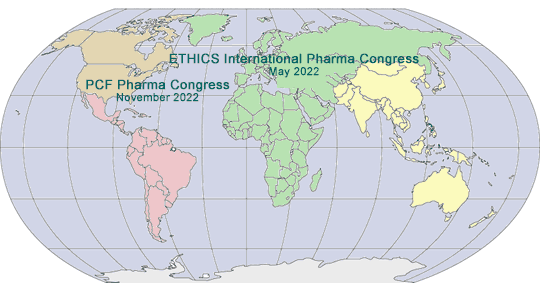
2022 GLOBAL PHARMA/MED DEVICE ETHICS & COMPLIANCE CONGRESSES
|
|
VIRTUAL FIFTEENTH INTERNATIONAL PHARMACEUTICAL AND MEDICAL DEVICE ETHICS & COMPLIANCE CONGRESS
|
Virtual Online Video Event Live and Archived
Sponsored by International Society of Healthcare Compliance Professionals (ETHICS)
Media Partner:
Policy & Medicine Compliance Update
May 17 - 20, 2022
www.International PharmaCongress.com
|
|
TWENTY-THIRD ANNUAL PHARMACEUTICAL AND MEDICAL DEVICE ETHICS & COMPLIANCE CONGRESS
|
Virtual Online Video Event Live and Archived
Sponsored by Pharmaceutical Compliance Forum
Media Partners:
Harvard Health Policy Review, Health Affairs and
Policy & Medicine Compliance Update
November 2022
www.PharmaCongress.com
|
|
|
|
CONGRESS EXHIBIT & SPONSORSHIP INFORMATION
|
|
For sponsorship and exhibit information, visit or contact Suzanne Tyler, Exhibit Manager, at (206) 244-4861 phone, (206) 319-5303 fax, or
exhibits@hcconferences.com.
|
|
FOR E-MAIL ADDRESS CHANGE, ADD OR DELETE REQUESTS
|
For changes or additions, please email your request to: listmgr@PharmaUpdateNewsService.com.
For removal of your e-mail address, please click the link below for "SafeUnsubscribe" to automatically remove your address from the list.
|
|
|

