|
|
|
Press Release: The Pharmaceutical Compliance Forum's Eighteenth Pharmaceutical and Medical Device Compliance Congress Features Enforcement Trends, Risk Assessments and Best Practices for Patient Assistance and Reimbursement Support
|
- Sponsored by the Pharmaceutical Compliance Forum
- November 6 - 8, 2017
- Onsite at the Mandarin Oriental, Washington, DC
- Online in Your Own Office or Home Live via the Internet with 24/7 Access for Six Months
- www.PharmaCongress.com
PRESS RELEASE
Phone: 800-503-7419
Email: registration@hcconferences.com
Website: www.PharmaCongress.com |
EARLY BIRD REGISTRATION
-- SAVE UP TO $200 --
|
Register by Friday, October 13, 2017 and save up to $200.
Click here to register.
|
|
FEATURING ENFORCEMENT TRENDS, RISK ASSESSMENTS AND BEST PRACTICES
FOR PATIENT ASSISTANCE AND REIMBURSEMENT SUPPORT BY
|

Jennifer Chillas, JD
Senior Corporate Counsel, Bristol-Myers Squibb, Princeton Pike, NJ |

Nereyda Garcia, JD
Global Head, Ethics and Compliance, Alnylam Pharmaceuticals; Former Senior Director, Compliance, Biogen Idec; Boston, MA
|

Laura G. Hoey, JD
Partner, Ropes & Gray LLP; Former Assistant US Attorney, US Attorney's Office, Eastern District of Arkansas, US Department of Justice; Chicago, IL |

Casey J. Horton, CFE
Director, Healthcare and Life Sciences Disputes, Regulatory, Compliance and Investigations, Navigant Consulting, Chicago, IL (Moderator)
|
|
|
WASHINGTON, DC USA -- PHARMA UPDATE NEWS SERVICE™ -- SEPTEMBER 26, 2017: The Pharmaceutical Compliance Forum's Eighteenth Pharmaceutical and Medical Device Compliance Congress, www.PharmaCongress.com, will be held November 6 - 8, 2017 at the Mandarin Oriental in Washington, DC. Registrants may attend the event in person or online (live webcast and archived for 6 months).
|
AGENDA-AT-A GLANCE
|
|
|
|
MONDAY, NOVEMBER 6, 2017
|
|
MORNING INVITATION-ONLY CCO ROUNDTABLE
|
|
|
|
MORNING PRE-CONFERENCES:
|
- Advanced Medical Affairs Compliance Issues
- The Basics of Managed Markets
- Medical Device Best Practice Compliance Updates
- Government Programs for the Compliance Officer
|
|
|
AFTERNOON OPENING PLENARY SESSION:
|
- OIG Compliance Update
- DOJ Evaluation of Corporate Compliance Programs
- DOJ, SEC and FBI FCPA Enforcement Update
- AUSA Roundtable
- Chief Compliance Officer Roundtable
- Networking Reception
|
|
|
TUESDAY, NOVEMBER 7, 2017
|
|
MORNING PLENARY SESSION:
|
- FDA Compliance Update
- Qui Tam Roundtable
- Demonstrating the Value of the Compliance Organization
|
|
MORNING MINI SUMMIT BLOCK A:
|
- Using Behavioral Economics to Improve Compliance Messaging Stickiness
- HCP Engagement: The Road to Proactive Risk Management
- Best Practices for Patient Assistance and Reimbursement Support
- Managed Market Considerations for Hub and Specialty Pharmacy Arrangements
- Compliance and Ethics Consideration in R&D
- Using Data Analytics to Tell the Compliance Effectiveness Story
- International Organization for Standardization (ISO) 37001
|
NETWORKING LUNCHEON AND
LUNCHEON MINI SUMMITS:
|
- FDA Regulation and Cutting Edge Technology
- Advancing Compliance: Pulling Critical Levers to Improve Effectiveness
- The Evolution of Compliance Programs after Wells Fargo
- Innovation and Compliance, They are not Mutually Exclusive
|
|
|
AFTERNOON MINI SUMMIT BLOCK B:
|
- Third Party Risk Management/Due Diligence Update
- Compliance in the Transactional Context
- Managing an Internal Investigation under CIA, DPA and Data Analytics Failure
- Recent FDA Guidance: Manufacturer Communications and Off-Label Memo
- Small and Mid-Sized Pharma and Device Companies Compliance Considerations
- Advanced Issues in Global Compliance
- Effective and Balanced Monitoring Program based on Risk
|
|
AFTERNOON MINI SUMMIT BLOCK C:
|
- Advanced Compliance Issues When Contracting with Third Parties
- The Next Generation of Agg Spend Solutions
- Cyber Security for Pharma and Medical Device Companies
- Pricing Considerations (Mylan Epipen, Marathon Pharmaceuticals, et al)
- Optimizing Data Collection for Risk Assessments, Monitoring, and Auditing
- Balancing Risks in Patient and Product Support
|
|
AFTERNOON CLOSING PLENARY SESSION:
|
- Role of Behavioral Economics in Ethics and Compliance
- PhRMA Priorities and Policy Initiatives
- Overview of the Policy and Politics of Pharma Pricing
- Rewarding Results: Value-Based Contracting for Biopharmaceuticals
|
|
|
WEDNESDAY, NOVEMBER 8, 2017
|
|
MORNING INDUSTRY-ONLY COMPLIANCE BEST PRACTICES THINK TANK
|
- (Industry-only session for pharmaceutical company compliance professionals and in-house counsel only)
|
|
|
|
KEYNOTE SPEAKERS
|

Thomas W. Abrams, RPh, MBA
Director, Office of Prescription Drug Promotion, US Food and Drug Administration, Silver Spring, MD |

Tom Hubbard, MPP
Vice President of Policy Research, NEHI (The Network for Excellence in Health Innovation); Former Executive Assistant for Economic Affairs, US Senator John Kerry; Former Deputy Director of Development, Massachusetts Governor Michael Dukakis; Cambridge, MA |

Mary E. Riordan, Esq.
Senior Counsel, Office of Counsel to the Inspector General, Office of Inspector General, US Department of Health and Human Services, Washington, DC
|

James Sheehan, JD
Chief, Charities Bureau, Attorney General of New York; Former Chief Integrity Officer, New York City Human Resources Administration; Former, NY Medicaid Inspector General; Former AUSA, US Attorney's Office, Eastern District of Pennsylvania; New York, NY |

Jim Stansel, JD
Executive Vice President, General Counsel and Corporate Secretary, PhRMA; Former Acting General Counsel, US Department of Health and Human Services; Washington, DC
|
|
|
KEYNOTE DOJ CIVIL AND CRIMINAL DIVISIONS ROUNDTABLE
|

Colin M. Huntley, JD
Assistant Director, Civil Division, Fraud Section, U.S. Department of Justice, Washington DC |

Pablo Quiñones, JD
Chief, Strategy, Policy and Training Unit, Fraud Section, Criminal Division, US Department of Justice; Former Assistant United States Attorney in the Criminal Division, US Attorney's Office for the Southern District of New York; Washington, DC |

Gejaa T. Gobena, JD
Partner, Hogan Lovells; Former Deputy Chief, Fraud Section, Criminal Division; Former Trial Attorney, Civil Division, Fraud Section, US Department of Justice; Washington, DC (Moderator)
|
|
|
KEYNOTE DOJ, SEC AND FBI FCPA ENFORCEMENT ROUNDTABLE
|

Charles Cain, Esq.
Deputy Chief, FCPA Unit, US Securities and Exchange Commission, Washington, DC |

Tarek Helou, Esq.
Assistant Chief, FCPA Unit, Fraud Section, Criminal Division, US Department of Justice, Washington, DC
|

Darryl Wegner, JD
Unit Chief, International Corruption Unit, FBI, Washington, DC |

Gary F. Giampetruzzi, Esq.
Partner, Paul Hastings; Former Vice President and Assistant General Counsel, Head of Government Investigations, Pfizer Inc.; New York, NY (Moderator)
|
|
|
KEYNOTE AUSA ROUNDTABLE
|

Jacob T. Elberg, JD
Chief, Health Care and Government Fraud Unit, United States Attorney's Office, District of New Jersey, US Department of Justice, Newark, NJ |

Jason Mehta, JD
Assistant US Attorney, United States Attorney's Office Middle District of Florida, US Department of Justice, Jacksonville, FL
|

Gregg Shapiro, JD
Assistant US Attorney, US Attorney's Office, District of Massachusetts, US Department of Justice, Boston, MA |

John T. Bentivoglio, Esq.
Partner, Skadden Arps LLP; Former Special Counsel for Healthcare Fraud, and Chief Privacy Officer, US Department of Justice; Washington, DC (Moderator)
|
|
|
KEYNOTE QUI TAM ROUNDTABLE
|

Amy L. Easton, JD
Attorney at Law, Phillips & Cohen, LLP, Former Senior Trial Counsel, Department of Justice, Washington, DC |

Neil V. Getnick, JD
Managing Partner, Getnick & Getnick LLP; Former Assistant District Attorney, Manhattan District Attorney's Office; New York, NY |

Michael A. Morse, JD
Partner, Pietragallo, Gordon, Alfano, Bosick & Raspanti; Former Assistant District Attorney, Philadelphia District Attorney's Office; Philadelphia, PA
|

Robert Patten, JD
President and Chief Executive Officer, Taxpayers Against Fraud and TAF Education Fund; Former Assistant Attorney General and Managing Attorney, Medicaid Fraud Division, Office of the Attorney General, Commonwealth of Massachusetts; Washington, DC |

Virginia "Ginny" A. Gibson, Esq.
Partner, Hogan Lovells LLP; Former First Assistant U.S. Attorney, Eastern District of Pennsylvania, US Department of Justice; Philadelphia, PA (Co-Moderator) |

Kathleen Meriwether, JD
Principal, Fraud Investigation & Dispute Services, Ernst & Young LLP; Former Assistant United States Attorney, Eastern District of Pennsylvania, US Department of Justice; Philadelphia, PA (Co-Moderator)
|
|
|
KEYNOTE DEMONSTRATING THE VALUE OF THE COMPLIANCE ORGANIZATION
|

Kathleen Boozang, JD LLM
Dean and Professor of Law, Seton Hall Law School, Newark, NJ |

Jonathon L. Kellerman
Executive Vice President, Global Chief Compliance Officer, Allergan PLC, New York, NY
|

Michael Shaw, JD
Vice President and Compliance Officer, US Pharmaceuticals, GlaxoSmithKline; Former Senior Counsel, Office of Inspector General, US Department of Health and Human Services; Former Member, PCF Executive Committee; Philadelphia, PA |

Erinn Hutchinson
Partner, Advisory Services, PwC, Philadelphia, PA (Moderator)
|
|
|
KEYNOTE CCO ROUNDTABLE
|

Jill Dailey, MBA, JD
Vice President and Chief Compliance Officer, Incyte; Former Assistant General Counsel and Asia Pacific Compliance Lead, Pfizer; Former Head of US Ethics and Compliance, Novartis; New York, NY |

Margaret K. Feltz, MA, JD
Vice President, Chief Compliance Officer, Ethics and Compliance, Purdue Pharma LP; Former Member, PCF Executive Committee; Stamford, CT
|

Arjun Rajaratnam, JD, MS
Chief Compliance Officer, Smith & Nephew; Former Compliance Officer, US Pharmaceuticals, GlaxoSmithKline; Former Member, PCF Executive Committee; Raleigh, NC |

Paul Silver
Principal, Regulatory & Compliance Life Sciences Leader, Deloitte Advisory, Deloitte, Atlanta, GA
|
|
|
FEATURED FACULTY
|

Sergio Alegre, JD
Vice President, Global Compliance, Osmotica Pharmaceutical Corp.; Former Executive Director Compliance, Pacira Pharmaceuticals, Inc.; New York, NY |

Anthony L. Alvizu
Managing Director, Global Risk & Investigations Practice, FTI Consulting, Chicago, IL |

Yogesh Bahl, CPA, MBA
Managing Director, Alix Partners, New York, NY
|

Ann Beasley, JD
Director, Navigant Consulting; Former Senior Vice President, Chief Compliance Officer, Biogen; Boston, MA |

Thomas W. Beimers, Esq.
Partner, Hogan Lovells; Former Senior Counsel for Administrative and Civil Remedies, Office of the Inspector General, Department of Health and Human Services; Minneapolis, MN |

Andy Bender, MS, MBA
President and Founder, Polaris, New York, NY
|

Raymond A. Bonner, JD
Partner and Chair, Food, Drug and Medical Device Compliance and Enforcement Practice, Sidley Austin LLP; Former Assistant US Attorney, US Attorney's Office, District of Maryland; Washington, DC |

Kenneth Borgerding, JD
Vice President, Chief Compliance Officer & Corporate Counsel, Lundbeck; Former Senior Counsel, Takeda; Chicago, IL |

Anthony Brennan, MBA
Senior Manager, Fraud Investigation and Dispute Services, Ernest & Young; Former Senior Director, Governance, Metrics and Reporting, Health Care Compliance, Johnson & Johnson; Iselin, NJ
|

Katherine Buckley, MBA
Principal, Pharmaceutical and Life Sciences Advisory Practice, PwC, Philadelphia, PA |

Terra Buckley
Senior Director, US HCC, I&I Franchise, Global Compliance, Celgene Corporation, Summit, NJ |

Jennifer Chillas, JD
Senior Corporate Counsel, Bristol-Myers Squibb, Princeton Pike, NJ
|

Robert F. Church, JD
Partner, Hogan Lovells US LLP; Former, Associate General Counsel/Executive Director, Amgen; Los Angeles, CA |

Andrew T. Clark, MBA
Partner/Principal, EY, Washington DC |

Kellie B. Combs, JD
Partner, Ropes & Gray, Washington, DC
|

Joseph Coniker
Principal, Enterprise Performance Management (EPM) Analytics, Grant Thornton LLP, Raleigh, NC |

Brian Conner
Vice President, Head of Corporate Compliance, Strongbridge BioPharma plc, Feasterville Trevose, PA |

Tom Costa, JD
Pharmaceutical Consultant, Sanofi Board of Directors; Former Vice President, US Compliance & Ethics, Bristol-Myers Squibb; Washington, DC
|

Clarissa Crain
Senior Manager, Life Sciences Regulatory and Operational Risk, Deloitte & Touche LLP, Philadelphia, PA |

David Cromley, JD
Associate Vice President, Merck, Philadelphia, PA |

Meenakshi Datta, JD
Partner, Sidley Austin LLP, Chicago, IL
|

BJ D'Avella
Senior Manager, Life Sciences Regulatory and Operational Risk, Deloitte & Touche LLP, Atlanta, GA |

Gary Del Vecchio
Health Care Compliance Officer Cardiovascular & Metabolism and Puerto Rico, The Janssen Pharmaceutical Companies of Johnson & Johnson; Former, Executive Director, US Pharmaceutical Compliance & Ethics, Bristol-Myers Squibb; Titusville, NJ |

Mark A. DeWyngaert, MBA, PhD
Managing Director, Life Sciences Regulatory and Operational Risk, Deloitte & Touche LLP, New York, NY
|

Sarah diFrancesca, JD
Partner, Health Care and Life Sciences Regulatory Practice, Cooley, LLP, New York, NY |

Michelle Drozd, ScM
Deputy Vice President, Policy and Research, Pharmaceutical Research & Manufacturers of America (PhRMA), Washington, DC |

Corey Dunbar
Senior Manager, Fraud Investigations & Dispute Services, EY, Iselin, NJ
|

Gildas Durand
Partner/Principal, Fraud Investigation and Internal Audit, Ernst & Young LLP, Miami, FL |

Liisa Eisenlohr, PhD, MBA
Associate Director, Navigant; Former Senior Director, Global Medical Information, Clovis Oncology; Former Clinical Scientist, Genentech; San Francisco, CA |

Jill Fallows-Macaluso, JD
Chief Compliance Officer and Vice President, Novo-Nordisk, Princeton, NJ
|

David J. Farber, JD
Partner, Healthcare Lobbyist and Litigator, King & Spalding LLP, Washington, DC |

Alison Fethke, JD
Counsel, Ropes & Gray LLP; Former Counsel, Legal, Regulatory & Compliance, AbbVie; Chicago, IL |

Nereyda Garcia, JD
Global Head, Ethics and Compliance, Alnylam Pharmaceuticals, Alnylam Pharmaceuticals; Former Sr. Director, Compliance, Biogen Idec; Boston, MA
|

Thomas M. Glavin, JD
Chief Compliance Officer for the Americas, Olympus Corporation of the Americas, Center Valley, PA |

Jonathan Glazier, JD, MBA
Senior Legal Counsel, Legal Compliance, Philips Electronics North America; Former Senior Director of Corporate Compliance and Privacy Officer, Fresenius Medical Care North America; Andover, MA |

Wendy C. Goldstein, JD
Partner, Health Care and Life Sciences Regulatory Practice, Cooley, LLP, New York, NY
|

Amy Gregory, CCEP
Director, Compliance for US Oncology, AstraZeneca, Gaithersburg, MD |

Pamela W. Guthrie, JD
Senior Counsel, Professional Affairs and Compliance, MicroPort Orthopedics, Arlington, TN |

David Hartenbaum, MD, MBA
Executive Director, Account Management, Merck, Philadelphia, PA
|

Lindsay Havern, JD
Vice President and Assistant General Counsel, Pfizer, New York, NY |

Justin Herring, JD
Assistant United States Attorney, United States Attorney's Office, District of New Jersey, US Department of Justice, Newark, NJ |

Laura G. Hoey, JD
Partner, Ropes & Gray LLP; Former Assistant US Attorney, US Attorney's Office, Eastern District of Arkansas, US Department of Justice; Chicago, IL
|

Casey J. Horton, CFE
Director, Healthcare and Life Sciences Disputes, Regulatory, Compliance and Investigations, Navigant Consulting, Chicago, IL |

Brian Van Hoy
Vice President, G & M Health LLC; Former Director, Ethics and Compliance, Eli Lilly; Bridgewater, NJ |

William Hrubes, MHA
Vice President, Chief Compliance Officer, ACell, Inc., Columbia, MD
|

William J. Hughes, Jr., JD, LLM
Principal, Porzio, Bromberg & Newman, PC; Former Assistant US Attorney and Trial Attorney, US Department of Justice; Morristown, NJ |

Jamil N. Jaffer, JD
Vice President for Strategy & Business Development, IronNet Cybersecurity, Adjunct Professor and Founder, National Security Law & Policy Program, Antonin Scalia Law School, George Mason University, Washington, DC |

Darren R. Jones, CIA
Managing Partner, Polaris Management Partners, New York, NY
|

Gary Keilty
Managing Director, FTI Consulting, Tampa, FL |

Anup Kharode, MBA
Principal, Pharmaceutical & Life Sciences R&D Advisory Services, PwC, Philadelphia, PA |

John Knighton, JD
Vice President, Head of Global Compliance, Orexigen Therapeutic; Former Vice President, CCO, MicroPort Medical (Group) Co., Ltd.; San Diego, CA
|

Keith M. Korenchuk, JD, MPH
Partner, Arnold & Porter LLP, Washington, DC |

Daniel A. Kracov, Esq.
Partner and Head, FDA and Healthcare Practice, Arnold & Porter, Washington, DC |

Holly Kramen, JD
Vice President, Global Compliance Officer, Circassia, Morrisville, NC
|

Monica Kwacinski, PharmD
Head of External Medical Affairs, Purdue Pharma LP, Stamford, CT |

Mark Lange, JD
Assistant General Counsel, Eli Lilly and Company, Indianapolis, IN |

Terri Ledva, MS
Healthcare Compliance Leader; Former Ethics & Compliance Leader, Iroko Pharmaceuticals; Former Associate Director, Ethics & Compliance, Bristol-Myers Squibb; Philadelphia, PA
|

Jonathan Levy, JD
Corporate Compliance Lead, Spark Therapeutics; Former Associate Director of Compliance and Ethics, US Pharmaceuticals, Bristol-Myers Squibb; Philadelphia, PA |

Maureen Lloyd
Director Pharmaceutical and Life Sciences, PwC; Former Executive Director, Medical - External Medical Communications, Pfizer Inc.; New York, NY |

Pamela Lonzer, MJ
US Medical Advisor, Shire; Former Associate Director, Global Medical Affairs Compliance, Baxalta; Former Assistant Director, R&D-Global Compliance, Astellas Pharma; Chicago, IL
|

Michael K. Loucks, JD
Partner, Skadden Arps LLP; Former Acting United States Attorney, District of Massachusetts, United States Department of Justice; Washington, DC |

Chia-Feng Lu, JD
Associate, Baker McKenzie, Washington, DC |

Seth H. Lundy, JD
Partner, King & Spalding, Washington, DC
|

Brian Miller, PhD
Director of Compliance and Ethics Training, Otsuka Pharmaceutical Companies, Senior Manager of Learning Technologies, AstraZeneca; Former Associate Director Learning Design & Technology, Merck; Philadelphia, PA |

Meghan Naas, MA
Senior Finance Manager, Compliance TESARO, Inc., Waltham, MA |

Michael O'Connor, MS
Former Senior Director, Global Head Compliance and Ethics Operations, Alexion Pharmaceuticals, Inc., New Haven, CT
|

Neil O'Flaherty, JD
Partner, Baker McKenzie, Washington, DC |

William P. Olsen
Principal, Practice Leader, Corporate Compliance, Forensic Advisory Services, Grant Thornton LLP, Arlington, VA |

John Patrick Oroho, JD
Executive Vice President, and Chief Strategy Officer, Porzio Life Sciences, LLC, Principal, Porzio, Bromberg & Newman PC, Morristown, NJ
|

Becky Osowski, MJ
Senior Manager, Fraud Investigation and Dispute Services Practice; Former US Healthcare Compliance Officer and Deputy Compliance Officer, Zimmer; Chicago, IL |

Amy Pawloski
Global Lead, Compliance Risk Mitigation and Monitoring Strategy, Bristol-Myers Squibb, Philadelphia, PA |

Paul J. Peterson, CPA, CIA, CFE, CFF
Senior Manager, Forensic Advisory Services, Grant Thornton, Arlington, VA
|

Kathryn Pryze, CCEP
Compliance Director, US Medical Affairs, and US Corporate Affairs, AstraZeneca, Wilmington, DE |

John Seungjoo Rah, JD
Partner, DLA Piper, Washington, DC |

Louis Ramos, JD
Partner, DLA Piper; Former Assistant General Counsel, Compliance Division, Pfizer; Former Assistant U.S. Attorney, US Attorney's Office, District of Columbia, US Department of Justice; Washington, DC
|

Kelly N. "Nikki" Reeves, MPA, JD
Partner, King & Spalding LLP, Washington, DC |

Beth Richardson
Director, Compliance, Horizon Pharma, Inc.; Former Compliance Director, CVS Health; Lake Forest, IL |

Jennifer Sanfilippo, JD
Vice President, Commercial Integrity Counsel, The Medicines Company, New York, NY
|

Jennifer Santos, JD
Head of Global Corporate Compliance Operations, Vertex Pharmaceuticals, Boston, MA |

Sue Seferian, JD
Health Care Compliance Officer, Johnson & Johnson, Titusville, NJ |

John D. Shakow, JD
Partner, King & Spalding LLP, Washington, DC
|

Brian Sharkey, JD
Porzio, Bromberg & Newman, PC, Morristown, NJ |

Greg Sherman, JD
Commercial Counsel, Gilead Sciences, Foster City, CA |

Michelle Shwery, MSc, MBA
Senior Director, Ethics and Compliance, Eli Lilly and Company, Indianapolis, IN
|

Laura Skinner
Senior Manager, Life Sciences Regulatory and Operational Risk, Deloitte & Touche LLP, Austin, TX |

Jon Smollen, MA, JD
Director, Center for Compliance and Ethics, Temple Law School; Former EVP and CCO, Endo; Former VP and CCO, Siemens Healthcare USA; Former VP, Commercial Excellence and Compliance, Wyeth; Philadelphia, PA |

Mariann Snyder, CCEP
Global Compliance Communications Lead, AstraZeneca, Wilmington, DE
|

Oliver Steck
Principal, Life Sciences Regulatory and Operational Risk, Deloitte & Touche LLP, New York, NY |

Tracy Strong, JD
Vice President, International Compliance Officer, Laboratory Corporation of America, Burlington, NC |

Cortnaye Swan
Sr. Manager, Deloitte & Touche LLP, Kansas City, MO
|

Jack Tanselle, MBA
Managing Director, Life Sciences Regulatory and Operational Risk, Deloitte & Touche LLP, Indianapolis, IN |

Ann-Marie Tejcek, MA
Senior Director Ethics & Compliance, USA/Canada, Eli Lilly and Company, Indianapolis, IN |

Bryan Timer
Associate Director, Data Analytics & Transparency, Merck, Allentown, PA
|

Andrew R. Van Haute, JD
Associate, Sidley Austin LLP; Former Associate General Counsel, AdvaMed; Washington, DC |

Julie Ritchie Wagner, JD
Assistant General Counsel, PhRMA; Former Senior Counsel, Office of Counsel to the Inspector General, US Department of Health and Human Services; Washington, DC |

Lisa Walkush
Principal, Advisory Services; National Life Sciences Sector Leader, Grant Thornton LLP, Philadelphia, PA
|

Caroline West, JD
Global Chief Compliance Officer, Olympus Corporation; Former Senior Vice President, Chief Compliance and Risk Officer, Shire; Former Vice President Global Legal Compliance, Aventis; Philadelphia, PA |

Matt Wetzel, JD
Vice President and Assistant General Counsel, Advanced Medical Technology Association (AdvaMed); Former Senior Counsel, Global Compliance and Ethics, Boston Scientific; Washington, DC |

Donna White, CCEP
Vice President, Contracts & Compliance, Chiesi USA, Inc.; Former Sr. Director, Contracts and Compliance, Cornerstone Therapeutics; Cary, NC
|

Seth B. Whitelaw, JD, LLM, SJD
President and Chief Executive Officer, Whitelaw Compliance Group, LLC, Editor, Life Science Compliance Update; Former Interim Chief Compliance Officer, Misonix, Inc.; West Chester, PA |

Jane H. Yoon, JD
Of Counsel, Litigation Department, Paul Hastings; Former Assistant United States Attorney, Health Care and Government Fraud Unit, US Attorney's Office for the District of New Jersey, US Department of Justice; New York, NY
|
|
|
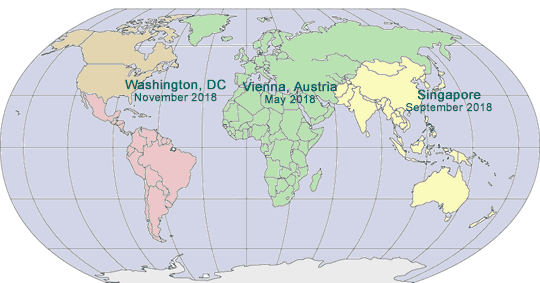
2017-2018 GLOBAL PHARMA COMPLIANCE CONGRESSES
|
|
ELEVENTH INTERNATIONAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
Sponsored by International Society of Healthcare Compliance Professionals (ETHICS)
Cosponsored by Pharmaceutical Compliance Forum (PCF)
May 15 - 17, 2017
Lisbon Marriott Hotel
Lisbon, Portugal
www.International PharmaCongress.com
|
|
SEVENTH ASIA PACIFIC PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
Sponsored by Asia Pacific Healthcare Industry Compliance Team
Cosponsored by International Society of Healthcare Ethics and Compliance Professionals (ETHICS) and Pharmaceutical Compliance Forum (PCF)
September 13 - 15, 2017
InterContinental Shanghai, Shanghai, China
www.Asian PharmaCongress.com
|
|
EIGHTEENTH ANNUAL PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
A Hybrid Conference and Internet Event
Sponsored by Pharmaceutical Compliance Forum (PCF)
November 6 - 8, 2017
Mandarin Oriental
Washington, DC
www.PharmaCongress.com
|
|
INDIAN PHARMACEUTICAL AND MEDICAL DEVICE COMPLIANCE CONGRESS
|
International Society of Healthcare Compliance Professionals (ETHICS)
Cosponsored by International Society of Healthcare Compliance Professionals (ETHICS) and Pharmaceutical Compliance Forum (PCF)
Spring 2018
Mumbai, India
Coming Soon
|
|
|
|
|
|
SPONSORED BY
|

The Pharmaceutical Compliance Forum (PCF) is a coalition of senior compliance professionals and legal counsel from more than 70 of the largest research-based pharmaceutical manufacturers. The PCF was founded in early-1999 by compliance professionals from the pharmaceutical industry to promote effective corporate compliance programs.
|
|
CO CHAIRS
|

Matthew D'Ambrosio, JD, MBA
Senior Vice President, Chief Compliance and Ethics Officer, Sunovion Pharmaceuticals Inc., Chair, Pharmaceutical Compliance Forum, Marlborough, MA
Sujata T. Dayal, JD
Vice President, Health Care Compliance & Privacy, Pharmaceuticals Group, Johnson & Johnson, Titusville, NJ

Jeffrey Kawalek, MBA
Senior Director, Ethics & Compliance, North America, Ipsen Biopharmaceuticals, Inc., New York, NY

Jennifer McGee, JD
Vice President and Chief Compliance Officer, Otsuka America Pharmaceutical, Inc., Rockville, MD

Margaret Sparks, JD
Associate Vice President, North America Ethics and Business Integrity, Sanofi, US, Bridgewater, NJ
|
|
GRANTORS:
|
|
PLATINUM
|
|
|
SILVER
|




|
|
BRONZE
|















|
|
MEDIA PARTNERS:
|



|
|
CONTINUING EDUCATION CREDITS:
|
Accounting Professionals: Approved for up to 18.5 NASBA CPE credits.
Attorney Credits: Pending Approval for PA MCLE credits.
Compliance Professionals: This education activity has been submitted to the Compliance Certification Board (CCB)® and is currently pending their review for approval of CCB CEUs.
Click here for more information.
|
|
PLANNING COMMITTEE
|
|
CO CHAIRS
|
Matthew D'Ambrosio, JD, MBA
Senior Vice President, Chief Compliance and Ethics Officer, Sunovion
Sujata T. Dayal, JD
Vice President, Health Care Compliance & Privacy, Pharmaceuticals Group, Johnson & Johnson
Jeffrey Kawalek, MBA
Senior Director, Ethics & Compliance, North America, Ipsen
Jennifer McGee, JD
Vice President and Chief Compliance Officer, Otsuka
Margaret Sparks, JD
Associate Vice President, North America Ethics and Business Integrity, Sanofi
|
|
MEMBERS
|
Tony Alvizu
FTI Consulting
Eric Baim, JD
Vice President, Head of Compliance US, Shire
Scott Bass, JD
Partner and Head, Global Life Sciences Team, Sidley Austin LLP
Yogesh Bahl, CPA, MBA
Managing Director, Alix Partners
John T. Bentivoglio, JD
Partner, Skadden Arps LLP
Thomas Beimers, JD
Partner, Hogan Lovells
Andy Bender, MS, MBA
President and Founder, Polaris
Kathleen M. Boozang, JD, LLM
Dean and Professor of Law, Seton Hall University School of Law
Michael R. Clarke, JD
Vice President, Corporate Compliance, Indivior Kris Curry, MBA, Principal, EY
Michael B. Dusseau
Vice President, Compliance Operations, Allergan PLC
Margaret K. Feltz, MA, JD
Vice President, Ethics and Compliance, Purdue Pharma LP
Gary F. Giampetruzzi, JD
Partner and Vice-Chair of Investigations, Paul Hastings
Wendy C. Goldstein, JD
Partner, Health Care and Life Sciences Regulatory Practice, Cooley, LLP
Steven Guymon
Senior Advisor, Global Ethics and Compliance Capabilities, Eli Lilly
Laura G. Hoey, JD
Partner, Ropes & Gray LLP
Erinn Hutchinson
Partner, Advisory Services, PwC
Mike Joachim, JD
Compliance Business Partner, Sanofi Genzyme
Jonathon L. Kellerman
Executive Vice President, Global Chief Compliance Officer, Allergan PLC
Shannon Kelley
Vice President, Head of North America Compliance, Sanofi
Daniel A. Kracov, JD
Partner and Head, FDA and Healthcare Practice, Arnold & Porter Kaye Scholer, LLP
Terri Ledva, MS
Senior Manager, Iroko Pharmaceuticals
Christine Longawa, MA, CPA, CFE
Associate Director, Navigant Consulting
Maureen McGirr, JD
Vice President Office of Ethics, Global Compliance Organization, Merck
Ed Nowicki, JD
Vice President and General Counsel, Pfizer, Inc. Neil O'Flaherty, Partner, Baker McKenzie
John Patrick Oroho, JD
Executive Vice President, and Chief Strategy Officer, Porzio Life Sciences, LLC
Lori Queisser
Senior Vice President and Global Chief Compliance Officer, Teva
Arjun Rajaratnam, JD, MS
Chief Compliance Officer, Smith & Nephew
David Ralston, JD, MPH
Senior Director, Associate General Counsel, Business Conduct, Gilead Sciences
Kelly N. “Nikki” Reeves, MPA, JD
Partner, King & Spalding
Michael Shaw, JD
Vice President and Compliance Officer, US Pharmaceuticals, GlaxoSmithKline
Paul Silver
Principal, Deloitte & Touche LLP
Carlos Tessi, MD, PhD
Vice President, Compliance, Shionogi
Donna Wachman
National Industry Marketing Leader, Grant Thornton LLP
Julie Wagner, JD
Assistant General Counsel, PhRMA
Seth B. Whitelaw, JD, LLM, SJD
President and Chief Executive Officer, Whitelaw Compliance Group, LLC
|
|
PARTICIPATION OPTIONS
|
|
TRADITIONAL ONSITE ATTENDANCE
|
Simply register, travel to the conference city and attend in person.
Pros: subject matter immersion; professional networking opportunities; faculty interaction

|
|
LIVE AND ARCHIVED WEBCAST PARTICIPATION
|
Watch the conference in live streaming video over the Internet and at your convenience at any time 24/7 for the six months following the event.
The archived conference includes speaker videos and coordinated PowerPoint presentations.
Pros: Live digital feed and 24/7 Internet access for next six months; Accessible in office, at home or anywhere worldwide with Internet access; Avoid travel expense and hassle; No time away from the office


|
|
WEBCAST INTERFACE SAMPLE
|
Click here for a sample stream
|
|
STAY CONNECTED
|


Tweet using #PharmaCongress
|
|
PAST GLOBAL PHARMA COMPLIANCE CONGRESSES
|

2007 - Brussels

2008 - Paris

2009 - Rome

2010 - Berlin

2011 - Istanbul

2011 - Singapore

2012 - Budapest

2012 - Shanghai

2012 - São Paulo

2013 - Madrid

2013 Kuala Lumpur

2014 - Dubai

2014 - Mexico City

2015 - Manila

2016 - Warsaw

2017 - Lisbon
|

 This site complies with the HONcode standard for trustworthy health information: This site complies with the HONcode standard for trustworthy health information:
verify here.


|

|
|
|
|
|